Abstract
Background
As an inhibitor of GSDMD, Disulfiram (DSL) can significantly inhibit cell pyroptosis. Cell pyroptosis plays an important role in renal fibrosis.
Methods
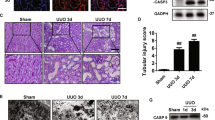
HK-2 cells were induced by Lps and ATP to form a pyroptosis model, and the cells were treated by DSL. CCK-8 detected the cell activity. Immunofluorescence (IF) detected the GSDMD. ELISA detected the expression of inflammatory cytokines. Flow cytometry and Western blot detected cell apoptosis and pyroptosis. Collagen type I kit detected collagen secretion, and western blot detected fibrosis marker protein expression. Then, a rat model of unilateral ureteral obstruction (UUO) was established. HE staining detected the degree of renal tissue injury, and Masson staining detected the degree of fibrosis. What’s more, the apoptosis level of tissue cells was detected by TUNEL. And the inflammatory factors in peripheral blood and renal tissue were detected by ELISA. Furthermore, the expression of GSDMD was detected by immunohistochemistry (IHC), and Western blot was used to detect the expression levels of apoptosis and pyroptosis-related proteins in tissues.
Results
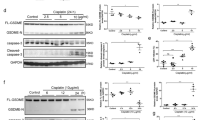
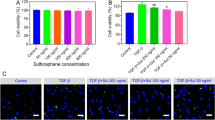
It was found that DSL can inhibit the cell membrane perforation of GSDMD-N by inhibiting the cleavage of GSDMD, hence, it inhibited the occurrence of inflammation, cell pyroptosis, and the fibrosis of HK-2 cells. But if the cell has already undergone pyroptosis, DSL does not provide significant prevention. In vivo experiment, it further verified that pretreated DSL had inhibited renal fibrosis injury.
Conclusion
Disulfiram can inhibit inflammation and fibrosis in renal fibrosis rats by inhibiting GSDMD.







Similar content being viewed by others
Availability of data and materials
The analyzed data set generated during the present study are available from the corresponding author on reasonable request.
References
Nastase MV, et al. Targeting renal fibrosis: Mechanisms and drug delivery systems. Adv Drug Deliv Rev. 2018;129:295–307.
Miao NJ, et al. Caspase-11 promotes renal fibrosis by stimulating IL-1beta maturation via activating caspase-1. Acta Pharmacol Sin. 2019;40(6):790–800.
Schnaper HW. Renal fibrosis. Methods Mol Med. 2005;117:45–68.
Sun YB, et al. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016;92(3):102–7.
Drawz P, Rahman M. Chronic kidney disease. Ann Intern Med. 2015;162(11):ITC1-16.
Kovacs SB, Miao EA. Gasdermins: Effectors of pyroptosis. Trends Cell Biol. 2017;27(9):673–84.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–54.
Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–42.
Wu M, et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol. 2018;478:115–25.
Guo H, et al. NLRP3 Deficiency attenuates renal fibrosis and ameliorates mitochondrial dysfunction in a mouse unilateral ureteral obstruction model of chronic kidney disease. Mediat Inflamm. 2017;2017:8316560.
Seo JB, et al. Gemigliptin attenuates renal fibrosis through down-regulation of the NLRP3 Inflammasome. Diabetes Metab J. 2019;43(6):830–9.
Lin J, et al. New insights into the mechanisms of pyroptosis and implications for diabetic kidney disease. Int J Mol Sci. 2020;21(19):7057.
Hu JJ, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–45.
Lu F, et al. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2020;235(4):3207–21.
Fang Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595.
Hutton HL, et al. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton). 2016;21(9):736–44.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–22.
Anders HJ, et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018;93(3):656–69.
Wang S, et al. Interleukin-22 ameliorated renal injury and fibrosis in diabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis. 2017;8(7):e2937.
Pulskens WP, et al. Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS ONE. 2014;9(1):e85775.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
XH contributed to conception and design, analysis and interpretation of the data critically revised the article for important intellectual content. YZ contributed to the design and analysis of the data, drafted and revised the manuscript. YZ and RZ substantially contributed to the conception and design, acquisition, analysis, and interpretation of data; drafted and critically revised the article for important intellectual content. All authors approved the final version of the article and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study protocol was approved by ethics committee of Nanjing Yimin Hospital. All of the procedures were in compliance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhang, R. & Han, X. Disulfiram inhibits inflammation and fibrosis in a rat unilateral ureteral obstruction model by inhibiting gasdermin D cleavage and pyroptosis. Inflamm. Res. 70, 543–552 (2021). https://doi.org/10.1007/s00011-021-01457-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01457-y