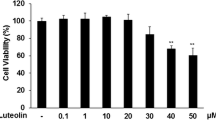
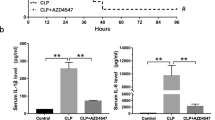
Abstract
Objective
Here, we used various approaches to investigate the suppressive role of daphnetin in LPS-induced inflammatory response, with the goal to understand the underlining molecular mechanism by which daphnetin regulated these processes.
Methods
We examined the survival rate and the lung injury in the mice model of LPS-induced endotoxemia. The production of pro-inflammatory factors including tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), IL-6, nitric oxide (NO), and prostaglandin E2 (PGE2) was measured by ELISA and nitrite analysis, respectively. The expression of inducible NO synthase (iNOS), cyclooxygenase 2 (COX-2), and the activation of signaling molecules was determined by immunoblotting. The production of reactive oxygen species (ROS) was measured by the ROS assay.
Results
In vivo study showed that daphnetin enhanced the survival rate and reduced the lung injury in mice with LPS-induced endotoxemia. Both in vivo and in vitro study showed that daphnetin prevented the production of pro-inflammatory factors including TNF-α, IL-1β, IL-6, NO, and PGE2 after LPS challenge. In Raw264.7 cells, we found that daphnetin reduced LPS-induced expression of iNOS and COX-2, and suppressed LPS-induced ROS production. In addition, we found that daphnetin suppressed the activation of JAK/STATs pathway and inhibited the nucleus import of STAT1 and STAT3.
Conclusions
Here, our results indicate that daphnetin shows anti-inflammatory properties, at least in part, through suppressing LPS-induced activation of JAK/STATs cascades and ROS production.




Similar content being viewed by others
Abbreviations
- DFN:
-
Daphnetin
- LPS:
-
Lipopolysaccharide
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- NO:
-
Nitric oxide
- PGE2:
-
Prostaglandin E2
- iNOS:
-
Inducible NO synthase
- COX-2:
-
Cyclooxygenase 2
- JAK:
-
Janus protein tyrosine kinase
- STAT:
-
Signal transducers and activators of transcription
- MAPKs:
-
Mitogen-activated protein kinases
- ROS:
-
Reactive oxygen species
- I.P. injection:
-
Intraperitoneal injection
References
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94.
Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–95.
Kang Y-J, Wingerd BA, Arakawa T, Smith WL. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. J Immunol. 2006;177:8111–22.
Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci. 1994;91:2046–50.
Manzi S, Wasko MCM. Inflammation-mediated rheumatic diseases and atherosclerosis. Ann Rheum Dis. 2000;59:321–5.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Davies M, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–35.
Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. The Journal of Immunology. 2004;172:20–4.
Noguchi S, Nakatsuka M, Konishi H, Kamada Y, Chekir C, Kudo T. Nafamostat mesilate suppresses NF-κB activation and NO overproduction in LPS-treated macrophages. Int Immunopharmacol. 2003;3:1335–44.
Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11.
Karin M. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene. 1999;18:6867–74.
Chan ED, Riches DW. IFN-γ + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 mapk in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441–50.
Chen B-C, Chen Y, Lin W. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–9.
Park EJ, Park SY, Joe E-h, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278:14747–52.
Okugawa S, Ota Y, Kitazawa T, Nakayama K, Yanagimoto S, Tsukada K, et al. Janus kinase 2 is involved in lipopolysaccharide-induced activation of macrophages. Am J Physiol Cell Physiol. 2003;285:C399–408.
Kisseleva T, Bhattacharya S, Braunstein J, Schindler C. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24.
Kovarik P, Mangold M, Ramsauer K, Heidari H, Steinborn R, Zotter A, et al. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J. 2001;20:91–100.
Wen Z, Zhong Z, Darnell JE. Maximal activation of transcription by Statl and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50.
Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int 2013; 2013.
Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10:3813–33.
R K, A L, E M HK. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–9.
Hoffmann A, Baltimore D. Circuitry of nucleus factor kappaB signaling. Immunological Reviews 2006;210:171–186.
Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9.
Qi Z, Yin F, Lu L, Shen L, Qi S, Lan L, et al. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm Res. 2013;62:845–55.
Pan X, Cao X, Li N, Xu Y, Wu Q, Bai J, et al. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Agents Actions. 2014;63:597–608.
Levy DE, Jr DJ. STATS: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62.
Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nucleus factor kappaB. Biochem J. 2002;367:e75683.
Pfitzner E, Kliem S, Baus D, Litterst CM. The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des. 2004;10:2839–50.
Caldow MK, Cameron-Smith D. JAK/STAT pathway: Heidelberg: Springer, 2012. pp. 495–497.
Jain N, Zhang T, Fong SL, Lim CP, Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK). Oncogene. 1998;17:3157–67.
Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24.
Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009;15:1702–7.
Devasagayam T, Tilak J, Boloor K, Sane KS, Ghaskadbi SS, Lele R. Free radicals and antioxidants in human health: current status and future prospects. Japi. 2004;52:4.
Aruoma OI, Grootveld M, Bahorun T. Free radicals in biology and medicine: from inflammation to biotechnology. Biofactors. 2006;27:1–3.
Kou X, Qi S, Dai W, Luo L, Yin Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int Immunopharmacol. 2011;11:1095–102.
Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res Int. 2013;2013:563–8.
Fylaktakidou KC, Ke HLD, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr Pharm Des. 2004;10:3813–33.
Yu W-w, Lu Z, Zhang H, Kang Y-h, Mao Y, Wang H-h, et al. Anti-inflammatory and protective properties of daphnetin in endotoxin-induced lung injury. J Agric Food Chem. 2014;62:12315–25.
Ferrero-Miliani L, Nielsen O, Ps, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147:227–35.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, Park DR, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–903.
Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, et al. Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax. 2015;70:740.
Andrey F, Lihua Y, Hua D, Hammock BD, Crofford LJ. Anti-inflammatory properties of prostaglandin E2: deletion of microsomal prostaglandin E synthase-1 exacerbates non-immune inflammatory arthritis in mice. Prostaglandins Leukot Essent Fatty Acids. 2013;89:351–8.
Macmicking J, Xie QA, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50.
Guo JY, Huo HR, Yang YX, Li CH, Liu HB, Zhao BS, et al. 2-methoxycinnamaldehyde reduces IL-1beta-induced prostaglandin production in rat cerebral endothelial cells. Biol Pharm Bulletin. 2006;29:2214–21.
Liu Z, Fan Y, Wang Y, Han C, Pan Y, Huang H, et al. Dipyrithione inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in macrophages and protects against endotoxic shock in mice. Febs Lett. 2008;582:1643–50.
Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci. 2001;98:8638–43.
T K, S B, CW S JB. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24.
Pellegrini S, Dusanter-Fourt I. The Structure, regulation and function of the Janus kinases (JAKs) and the signal transducers and activators of transcription (STATs). Eur J Biochem. 1997;248:615–33.
Galdiero M, Vitiello M, D’Isanto M, Raieta K, Galdiero E. STAT1 and STAT3 phosphorylation by porins are independent of JAKs but are dependent on MAPK pathway and plays a role in U937 cells production of interleukin-6. Cytokine. 2006;36:218–28.
Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–72.
Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Investig. 2001;107:7.
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:728–49.
Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol. 2006;72:1493–505.
Kamezaki K, Shimoda K, Numata A, Matsuda T, Nakayama KI, Harada M. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int Immunol. 2004;16:1173–9.
Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:1640–52.
Madamanchi N, Li S, Patterson C, Runge M. Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler Thromb Vasc Biol. 2001;21:321–6.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81471557, 31571166, 81671565, and 31500628) a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 164320H106) and Jiangsu Major Nature Science Foundation of High Education (No. 12KJA180006). We thank lab members for their encouragements.
Author contributions
Conceived and designed the experiments: ZMY LS. Performed the experiments: LS. Analysis the data: ZMY L. Lan LS L. Luo TZ JW XMS. Contributed reagents/materials/analysis tools: ZMY L. Lan L. Luo LS. Wrote the paper: LS ZMY L. Luo.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Shen, L., Zhou, T., Wang, J. et al. Daphnetin reduces endotoxin lethality in mice and decreases LPS-induced inflammation in Raw264.7 cells via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 66, 579–589 (2017). https://doi.org/10.1007/s00011-017-1039-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1039-1