Abstract
Objective
Although luteolin has shown to have anti-inflammatory action, no report is available whether luteolin inhibits HMGB1 and protects acute lung injury (ALI) in endotoxin rodents. We hypothesized that HO-1 induction by luteolin might play a crucial role for inhibition of pro-inflammatory mediators including HMGB1 through MAPK signaling in LPS-induced RAW264.7 cells, and it ameliorates ALI of endotoxin mice.
Methods
The effects of luteolin on the production of pro-inflammatory mediators in LPS-activated RAW264.7 cells and LPS-injected mice were evaluated. The mechanisms were investigated using various signal inhibitors.
Results
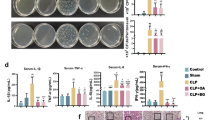
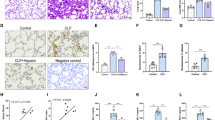
Luteolin significantly increased HO-1 expression through ERK1/2 signaling in a time- and concentration-dependent manner. Indeed, luteolin inhibited pro-inflammatory mediators (HMGB1, iNOS/NO, COX-2, and NF-κB activity) in LPS-activated RAW264.7 cells. In addition, PD98059, an ERK1/2 inhibitor, treatment failed to inhibit production of these pro-inflammatory mediators by luteolin. Interestingly, luteolin augmented HO-1 induction through Ca2+ influx in RAW264.7 cells. Administration of luteolin significantly inhibited plasma HMGB1 level, and iNOS expression in the lung that resulted in a significant reduction of ALI in endotoxin mice that was reversed by a HO-1 inhibitor, ZnPPIX.
Conclusion
Therefore, we conclude that luteolin has a great potential for treatment of ALI and related diseases, where HMGB1 is a therapeutic target.





Similar content being viewed by others
Abbreviations
- HMGB1:
-
High-mobility group box 1
- HO-1:
-
Heme oxygenase-1
- ALI:
-
Acute lung injury
- ZnPPIX:
-
Zinc protoporphyrin IX
- ERK1/2:
-
Extracellular signal-regulated protein kinase
- JNK:
-
c-Jun NH2-terminal kinase
- NF-κB:
-
Nuclear factor-κB
- MAPK:
-
Mitogen-activated protein kinase
- NO:
-
Nitric oxide
- LPS:
-
Lipopolysaccharide
- PAMP:
-
Pathogen-associated molecular pattern
- DAMP:
-
Damage-associated molecular pattern
- Nrf2:
-
Nuclear factor (erythroid-derived 2)-like 2
- ARE:
-
Antioxidant response element
- MTT:
-
Thiazolyl blue tetra-zolium bromide
- DMSO:
-
Dimethyl sulfoxide
- PVDF:
-
Polyvinylidene difluoride
- PG:
-
Prostaglandin
- IL:
-
Interleukin
- CO:
-
Carbon monoxide
- PI3K:
-
Phosphoinositide 3-kinase
References
Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13(6):701–11.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5.
Hung YL, Fang SH, Wang SC, Cheng WC, Liu PL, Su CC, et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci Rep. 2017;7:46299.
Lee W, Yoon EK, Kim KM, Park DH, Bae JS. Antiseptic effect of vicenin-2 and scolymoside from cyclopia subternata (honeybush) in response to HMGB1 as a late sepsis mediator in vitro and in vivo. Can J Physiol Pharmacol. 2015;93(8):709–20.
Zhao F, Fang Y, Deng S, Li X, Zhou Y, Gong Y, et al. Glycyrrhizin protects rats from sepsis by blocking HMGB1 signaling. Biomed Res Int. 2017;2017:9719647.
Yang R, Tenhunen J, Tonnessen TI. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis. Int J Inflamm. 2017;2017:1817564.
Yu Y, Yang Y, Bian Y, Li Y, Liu L, Zhang H, et al. Hydrogen gas protects against intestinal injury in wild type but not NRF2 knockout mice with severe sepsis by regulating HO-1 and HMGB1 release. Shock. 2017;48(3):364–70.
Tsoyi K, Nizamutdinova IT, Jang HJ, Mun L, Kim HJ, Seo HG, et al. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-beta/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock. 2010;34(6):608–14.
Yang H, Zhao P, Tian S. Clopidogrel protects endothelium by hindering TNFalpha-induced VCAM-1 expression through CaMKKbeta/AMPK/Nrf2 pathway. J Diabetes Res. 2016;2016:9128050.
Wu PS, Yen JH, Kou MC, Wu MJ. Luteolin and apigenin attenuate 4-hydroxy-2-nonenal-mediated cell death through modulation of UPR, Nrf2-ARE and MAPK pathways in PC12 cells. PLoS One. 2015;10(6):e0130599.
Kim JH, Park GY, Bang SY, Park SY, Bae SK, Kim Y. Crocin suppresses LPS-stimulated expression of inducible nitric oxide synthase by upregulation of heme oxygenase-1 via calcium/calmodulin-dependent protein kinase 4. Mediat Inflamm. 2014;2014:728709.
Liu CW, Lin HW, Yang DJ, Chen SY, Tseng JK, Chang TJ, et al. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-kappaB and activation of HO-1. Free Radic Biol Med. 2016;95:180–9.
Paredes-Gonzalez X, Fuentes F, Jeffery S, Saw CL, Shu L, Su ZY, et al. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm Drug Dispos. 2015;36(7):440–51.
Kim S, Chin YW, Cho J. Protection of cultured cortical neurons by luteolin against oxidative damage through inhibition of apoptosis and induction of heme oxygenase-1. Biol Pharm Bull. 2017;40(3):256–65.
Xiong J, Wang K, Yuan C, Xing R, Ni J, Hu G, et al. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med. 2017;39(1):113–25.
Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep. 2017;7(1):5850. https://doi.org/10.1038/s41598-017-06205-z.
Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14(11):2057–70.
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2.
Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu HB, et al. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol Appl Pharmacol. 2012;265(2):229–40.
Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72(9):1102–13.
Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through toll-like receptors. J Endotoxin Res. 2000;6(6):453–7.
Kashio M, Sokabe T, Shintaku K, Uematsu T, Fukuta N, Kobayashi N, et al. Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci USA. 2012;109(17):6745–50.
Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14(7):738–47.
Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol. 2010;184(5):2386–93.
Di A, Gao XP, Qian F, Kawamura T, Han J, Hecquet C, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol. 2011;13(1):29–34.
Qian X, Numata T, Zhang K, Li C, Hou J, Mori Y, et al. Transient receptor potential melastatin 2 protects mice against polymicrobial sepsis by enhancing bacterial clearance. Anesthesiology. 2014;121(2):336–51.
Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124(11):4926–40.
Acknowledgements
We thank Mr. Min S. Park for technical assistance. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2016R1A2B4008471).
Author information
Authors and Affiliations
Contributions
Conceived of or designed study: Chang KC. Performed research: Park EJ. Analyzed data: Kim HJ and Chang KC. Contributed new methods or models: Park EJ and Kim YM. Wrote the paper: Chang KC.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Additional information
Communicated by John Di Battista.
Rights and permissions
About this article
Cite this article
Park, E.J., Kim, Y.M., Kim, H.J. et al. Luteolin activates ERK1/2- and Ca2+-dependent HO-1 induction that reduces LPS-induced HMGB1, iNOS/NO, and COX-2 expression in RAW264.7 cells and mitigates acute lung injury of endotoxin mice. Inflamm. Res. 67, 445–453 (2018). https://doi.org/10.1007/s00011-018-1137-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1137-8