Abstract
Objectives
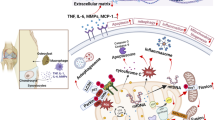
To investigate the expression of Sam68 in articular cartilage of knee osteoarthritis (OA) and the relationship between Sam68 and NF-κB activation and apoptosis signaling in OA articular chondrocytes.
Methods
Sam68 expression in normal and osteoarthritic cartilage was assessed by immunohistochemistry and RT-PCR on both meniscal/ligamentous injury (MLI)-induced OA rat model and the clinical human OA cartilage tissues. Sam68 expression in chondrocytes under tumor necrosis factor-alpha (TNF-α) stimuli was also assessed by immunoblot. Inhibiting Sam68 in chondrocytes under TNF-α stimuli was conducted using small interfering RNA (siRNA) and its influence on the expression of apoptotic marker and catabolic genes was examined by immunoblot. The mechanism of how Sam68 stimulates NF-κB activity was determined by co-immunoprecipitation and immunoblot analysis of nuclear and cytoplasmic fractions of TNF-α-treated chondrocytes for p65 and Sam68.
Results
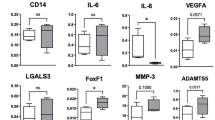
Sam68 expression was increased in OA cartilage tissues and chondrocytes under TNF-α stimuli. Inhibition of Sam68 by siRNA significantly decreased the expression of apoptotic markers (cleaved caspase-3 and cleaved PARP) in chondrocytes following TNF-α-stimulation. Sam68 knockdown suppressed Iκ-B degradation and p65 nuclear transportation in TNF-α-treated chondrocytes, indicating a suppressed NF-κB activation. Upon TNF-α exposure, the nuclear transportation of Sam68 and its interaction with p65 was detected in chondrocytes. Furthermore, Sam68 knockdown also alleviated the TNF-α-induced catabolic marker (MMP13, ADAMTS5, iNOS and IL-6) expression.
Conclusions
The highly expressed Sam68 promotes NF-κB signaling activation, catabolic gene expression and cellular apoptosis in TNF-α-treated chondrocytes, which may provide better insights into the pathophysiology of OA and a potential target for its treatment.




Similar content being viewed by others
References
Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Investig. 1989;84:678–85.
Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6.
Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–9.
Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–62.
Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis–structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391–9.
Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–8.
Todd Allen R, Robertson CM, Harwood FL, Sasho T, Williams SK, Pomerleau AC, et al. Characterization of mature vs aged rabbit articular cartilage: analysis of cell density, apoptosis-related gene expression and mechanisms controlling chondrocyte apoptosis. Osteoarthr Cartil. 2004;12:917–23.
Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–206.
Shi J, Schmitt-Talbot E, DiMattia DA, Dullea RG. The differential effects of IL-1 and TNF-alpha on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm Res. 2004;53:377–89.
Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11:599–613.
Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224.
Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–21.
Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;1653:73–86.
Najib S, Martin-Romero C, Gonzalez-Yanes C, Sanchez-Margalet V. Role of Sam68 as an adaptor protein in signal transduction. Cell Mol life sci CMLS. 2005;62:36–43.
Ramakrishnan P, Baltimore D. Sam68 is required for both NF-kappaB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43:167–79.
Hamada D, Sampson ER, Maynard RD, Zuscik MJ. Surgical induction of posttraumatic osteoarthritis in the mouse. Methods Mol Biol. 2014;1130:61–72.
Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13:632–41.
Sampson ER, Beck CA, Ketz J, Canary KL, Hilton MJ, Awad H, et al. Establishment of an index with increased sensitivity for assessing murine arthritis. J Orthop Res. 2011;29:1145–51.
Mainil-Varlet P, Schiavinato A, Ganster MM. Efficacy evaluation of a new hyaluronan derivative HYADD 4-G to maintain cartilage integrity in a rabbit model of osteoarthritis. Cartilage. 2013;4:28–41.
Yui N, Yoshioka H, Fujiya H, Musha H, Beppu M, Karasawa R, et al. The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int J Mol Sci. 2014;15:14921–34.
Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18(Suppl 3):S17–23.
Abedian R, Willbold E, Becher C, Hurschler C. In vitro electro-mechanical characterization of human knee articular cartilage of different degeneration levels: a comparison with ICRS and Mankin scores. J Biomech. 2013;46:1328–34.
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J, et al. Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity. Neurochem Int. 2012;61:1021–35.
Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr Cartil. 2011;19:222–32.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, No. 2012CB822104); National Natural Science Foundation of China (31170766, 31500647, 81171140); Key Project Natural Science Foundation of Jiangsu University and College (No. 15KJA310003); the Natural Science Foundation of Jiangsu Province (SBK2015041233); Nantong City Social Development Projects funds (HS2012032); a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); Development Fund for Collaborative Innovation Center of Glycoscience of Shandong University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jason J. McDougall.
L. Xu and C. Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, L., Sun, C., Zhang, S. et al. Sam68 Promotes NF-κB Activation and Apoptosis Signaling in Articular Chondrocytes during Osteoarthritis. Inflamm. Res. 64, 895–902 (2015). https://doi.org/10.1007/s00011-015-0872-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0872-3