Abstract
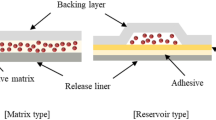
Development of an impeccable pharmaceutical product is always challenging in terms of process development and the product quality. Formulations meant for transdermal applications are sophisticated systems employing a plethora of constituents and using the traditional hit and trial approach of formulation design will lead to a troublesome job. This chapter enables one to understand about the various critical quality attributes and critical material attributes that affect the quality target profile of the transdermal product. Transdermal drug delivery systems (TDDS) are specially designed systems that are meant to deliver therapeutic agents across the skin of the patient for systemic effects. These systems are a type of controlled release drug delivery systems that tend to deliver therapeutic agents at a fixed rate over a protracted period of time. Quality by Design (QbD) being a scientific method that take into account various risk factors (risk assessment), quality target product profile (QTPP), critical quality attributes (CQA), and a control strategy for the process and product development. QbD aims at development of a TDDS product to deliver the optimum amount of drug across the skin while minimizing the amount of drug load, thus resulting in the least possible amount of residual drug substance. QbD leads to better understanding of the product and manufacturing process that contributes to profound assessment of the effects of variations in raw materials and the manufacturing process on drug product quality.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Raza K, Singh B, Singal P, Wadhwa S, Katare OP (2013) Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf B Biointerfaces 105:67–74
Garg V, Singh H, Bhatia A, Raza K, Singh SK, Singh B et al (2017) Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech 18(1):58–71
Singh B, Kumar R, Ahuja N (2005) Optimizing drug delivery systems using systematic “design of experiments.” Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst 2005:27–105
Sharma G, Thakur K, Raza K, Singh B, Katare OP (2017) Nanostructured lipid carriers: a new paradigm in topical delivery for dermal and transdermal applications. Crit Rev Ther Drug Carrier Syst 34(4):355–386
Shingade GM (2012) Review on: recent trend on transdermal drug delivery system. J Drug Deliv Ther 2:1
Wokovich AM, Prodduturi S, Doub WH, Hussain AS, Buhse LF (2006) Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur J Pharm Biopharm 64:1–8
Drugs@FDA: FDA-Approved Drugs [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=017874
Zecuity (Sumatriptan Iontophoretic Transdermal System): Uses, Dosage, Side Effects, Interactions, Warning [Internet]. [cited 2020 Jul 24]. Available from: https://www.rxlist.com/zecuity-drug.htm
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/anda/98/89884_Nitroglycerin Transdermal_Admindocs.pdf
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202513s003lbl.pdf
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/19813s039lbl.pdf
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021829lbl.pdf
Inactivated Influenza Vaccine Delivered by Microneedle Patch or by Hypodermic Needle - Full Text View - ClinicalTrials.gov [Internet]. [cited 2020 Jul 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT02438423
Mirataz (mirtazapine transdermal ointment) - Veterinarians | FDA [Internet]. [cited 2020 Jul 24]. Available from: https://www.fda.gov/animal-veterinary/product-safety-information/mirataz-mirtazapine-transdermal-ointment-veterinarians
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212268s000lbl.pdf
(No Title) [Internet]. [cited 2020 Jul 24]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204017s000lbl.pdf
Fda, Cder, hillsi. Transdermal and topical delivery systems-product development and quality considerations guidance for industry DRAFT GUIDANCE [Internet]. [cited 2020 Jun 21]. Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
Prausnitz MR, Langer R, Author NB (2008) Transdermal drug delivery NIH public access author manuscript. Nat Biotechnol 26(11):1261–1268
Sharma G, Dhankar G, Thakur K, Raza K, Katare OP (2016) Benzyl benzoate-loaded microemulsion for topical applications: enhanced dermatokinetic profile and better delivery promises. AAPS PharmSciTech 17(5):1221–1231
Bandyopadhyay A. Transdermal drug delivery system-quality by design approach. 2017. Available from http://transdermalspecialties.com/home.html
Fda, Cder, ocheltreet. Guidance for Industry Residual Drug in Transdermal and Related Drug Delivery Systems [Internet]. 2011 [cited 2020 Jun 21]. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
Garg NK, Sharma G, Singh B, Nirbhavane P, Tyagi RK, Shukla R et al (2017) Quality by Design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): an improved dermatokinetic profile for inflammatory disorder(s). Int J Pharm 517(1–2):413–431
Dhingani A, Patel J, Garala K, Raval M, Dharamsi A (2014) Quality by design approach for development of W/O type microemulsion-based transdermal systems for atenolol. J Dispers Sci Technol 35(5):619–640
Akhlaq M, Arshad MS, Mudassir AM, Hussain A, Kucuk I, Haj-Ahmad R et al (2016) Formulation and evaluation of anti-rheumatic dexibuprofen transdermal patches: a quality-by-design approach. J Drug Target 24(7):603–612
Jain S, Patel N, Madan P, Lin S (2015) Quality by design approach for formulation, evaluation and statistical optimization of diclofenac-loaded ethosomes via transdermal route. Pharm Dev Technol 20(4):473–489
Ahmed OAA, Kurakula M, Banjar ZM, Afouna MI, Zidan AS (2015) Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J Pharm Sci 104(6):2062–2075
Bakonyi M, Berkó S, Kovács A, Budai-Szűcs M, Kis N, Erős G et al (2018) Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J Drug Deliv Sci Technol 44:136–145
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mishra, M., Raza, K. (2021). Design of Experiments for the Development of Transdermal Drug Products. In: Beg, S. (eds) Design of Experiments for Pharmaceutical Product Development. Springer, Singapore. https://doi.org/10.1007/978-981-33-4351-1_4
Download citation
DOI: https://doi.org/10.1007/978-981-33-4351-1_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4350-4
Online ISBN: 978-981-33-4351-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)