Abstract
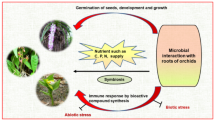
Orchids are considered to be the most highly differentiated and horticulturally important plants. Additionally, orchids have been used as traditional medicines in many countries since ancient times. Different organs of orchid plants, such as leaves, stems, and bulbs, contain various biologically active substances such as alkaloids, phenolics, terpenoids, and derivatives thereof. These bioactive compounds are secondary metabolites synthesized from primary metabolites of plants. To improve the utility of orchids, it is important to identify the pharmacological function of these plants. Moreover, the establishment of technologies for the large-scale production of a biomass of orchid plants using field cultivation or biotechnological methods is needed to prevent the overaccumulation of these plants in the natural state, which would ultimately result in these plants being enlisted as endangered species. Among various factors affecting the in vitro culture of medicinal orchids, growth regulators, light, sugar and activated charcoal are the most important. To establish a successful mass production system, it is necessary to determine the optimal concentration at which these factors maximize the production of biomass and bioactive compounds. In this chapter, we provide an overview of medicinal orchids and review recent studies on the in vitro production of biomass and bioactive compounds from these plants.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bhattacharyya P, Van Staden J (2016) Ansellia africana (leopard orchid): a medicinal orchid species with untapped reserves of important biomolecules – a mini review. S Afr J Bot 106:181–185
Bhattacharyya P, Kumaria S, Tandon P (2016) High frequency regeneration protocol for Dendrobium nobile: a model tissue culture approach for propagation of medicinally important orchid species. S Afr J Bot 2014:232–243
Bhummaphan N, Chanvorachote P (2015) Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid Based Complement Alternat Med 2015:836564
Brault M, Maldiney R (1999) Mechanisms of cytokinin action. Plant Physiol Biochem 37:405–412
Chai ML, Xua CJ, Senthil KK, Kim JY, Kim DH (2002) Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by agrobacterium tumefaciens. Sci Hortic 96:213–224
Chen JT, Chan WC (2004) TIBA affects the induction of direct somatic embryogenesis from leaf explants of Oncidium. Plant Cell Tissue Organ Cult 79:315–320
Chen JT, Chang WC (2000) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci 160:87–93
Chen WC, Lai YS, Lin SH, Lu KH, Lin YE, Panyod S, Ho CT, Sheen LY (2016) Anti-depressant effects of Gastrodiaelata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J Ethnopharmacol 182:190–199
Chou LC, Chang DC (2004) Asymbiotic and symbiotic seed germination of Anoectochilus formosanus and Haemaria discolor and their F1 hybrids. Bot Bull Acad Sin 45:143–147
Choury Z, Meschini R, Dell’Orso A, Fardusi MJ, Mugnozza GS, Kuzminsky E (2018) Plant Cell Tissue Organ Cult 132:535–543
Chow HT, Hsieh WC, Chang CS (1982) In vitro propagation of Anoectochilus formosanus. J Sci Eng 19:55–166
Cui HY, Murthy HN, Moh SH, Cui Y, Lee EJ, Paek KY (2014a) Protocorm culture of Dendrobium candidum in balloon type bubble bioreactors. Biochem Eng J 88:26–29
Cui HY, Murthy HN, Moh SH, Cui YY, Lee EJ, Paek KY (2014b) Production of biomass and bioactive compounds in protocorm cultures of Dendrobium candidum Wall ex Lindl. Using balloon type bubble bioreactors. Ind Crop Prod 53:28–33
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
Divakaran M, Babu KN (2009) Micropropagation and in vitro conservation of Vanilla (Vanilla planifolia Andrews). In: Jain SM, Saxena PK (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Springer, New York, pp 129–138
Du XM, Sub NY, Irino N, Shoyama Y (2000) Glycosidic constituents from in vitro Anoectochilus formosanus. Chem Pharm Bull 48:1803–1804
Dumas E, Monteuuis O (1995) In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tissue Organ Cult 40:231–235
Economou AS, Read PE (1986) Influence of light duration and irradiance on micropropagation of a hardy deciduous azalea. J American Soc Agricult 111:146–149
Gutiérrez RMP (2010) Orchids: a review of uses in traditional medicine, its phytochemistry and pharmacology. J Med Plant Res 4:592–638
Han YJ, Je JH, Kim SY, Ahn SM, Kim HN, Kim YR, Choi YW, Shin HK, Choi BT (2014) Gastrodiaelata shows neuroprotective effects via activation of PI3K signaling against oxidative glutamate toxicity in HT22 cells. Am J Chin Med 4:1007–1019
Ho CK, Chen CC (2003) Moscatilin from the orchid Dendrobiumloddigesii is a potential anticancer agent. Cancer Investig 21:729–736
Honda H, Liu C, Kobayashi T (2001) Large scale plant propagation. In: Scheper T (ed) Advances in biochemical engineering/biotechnology, vol 72. Springer-Verlag, Berlin, Heidelberg, pp 157–182
Hossain MM (2011) Therapeutic orchids: traditional uses and recent advances – an overview. Fitoterapia 82:102–140
Huang CH, Chung JP (2011) Efficient indirect induction of protocorm-like bodies and shoot proliferation using field-grown axillary buds of a Lycaste hybrid. Plant Cell Tissue Organ Cult 106:31–38
Huang K, Li Y, Tao S, Wei G, Huang Y, Chen D, Wu C (2016) Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules 21:E701
Irvani N, Solouki M, Omidi M, Zare AR, Shahnazi S (2010) Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tissue Organ Cult 100:293–299
Jang HR, Lee HJ, Park BJ, Pee OJ, Paek KY, Park SY (2016) Establishment of embryogenic cultures and determination of their bioactive properties in Rosa rugosa. Hortic Environ Biotechnol 57:291–298
Ket NV, Hahn EJ, Park SY, Chakrabarty D, Paek KY (2004) Micropropagation of an endangered orchid Anoectochilus formosanus. Biol Plant 48:339–344
Kim HJ, Moon KD, Oh SY, Kim SP, Lee SR (2001) Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus. Neurosci Lett 314:65–68
Lee GH, Kim HR, Han SY, Bhaandary B, Kim DS, Kim MG, So BO, Kim SY, Jo KS, Lee BH, Seos HN, Chae SW, Chae HJ (2012) Gastrodia elata Blume and its pure compounds protect BV-2 microglial-derived cell lines against β-amyloid: the involvement of Gastrodia elata P78 and CHOP. Biol Res 45:403–410
Lee JG, Moon SO, Kim SY, Yang EJ, Min JS, An JH, Choi EA, Liu KH, Park EJ, Lee HD, Song KS (2015) Rapid HPLC determination of gastrodin in Gastrodiae Rhizoma. J Korean Soc Appl Biol Chem 58:409–413
Lin JM, Lin CC, Chiu HF, Yang JJ, Lee SG (1993) Evaluation of the anti-inflammatory and liver protective effects of Anoectochilus formosanus, Ganderma lucidum and Gynostemma pentaphyllum. Am J Clin Med 11:59–69
Linington IM (1991) In vitro propagation of Dipterocarpus alatus and Dipterocarpus intricatus. Plant Cell Tissue Organ Cult 27:81–88
Martin KP (2002) Rapid propagation of Holostemmaada-kodienSchult., a rare medicinal plant, through axillary bud multiplication and indirect organogenesis. Plant Cell Rep 21:112–117
Mensuali-Sodia A, Panizza M, Serra G, Tognon F (1993) Involvement of activated charcoal in the modulation of abiotic and biotic ethylene levels in tissue cultures. Sci Hortic 54:49–57
Miller CO, Skoog F, Von Saltza MH, Strong F (1955) Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc 77:1392
Miller CO, Skoog F, Okomura FS, von Saltza MH, Strong FM (1956) Isolation, structure and synthesis of kinetin, a substance promoting cell division. J Am Chem Soc 78:1345–1350
Murashige T (1974) Plant propagation through tissue cultures. Ann Rev Plant Physiol 25:135–166
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tabacco tissue culture. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1–16
Murthy HN, Paek KY, Park SY (2018) Micropropagation of orchids by using bioreactor technology. In: Lee YI, Yeung EC (eds) Orchid propagation: from laboratories to greenhouses-methods and protocols. Springer, New York, pp 195–208
Naing AH, Chung JD, Park IS, Lim KB (2011) Efficient plant regeneration of the endangered medicinal orchid, Coelogyne cristata using protocorm-like bodies. Acta Physiol Plant 33:659
Nayak NR, Patnaik S, Rath SP (1997) Direct shoot regeneration from foliar explants of an epiphytic orchid Acampe praemorsa (Roxb.) Blatter and McCann. Plant Cell Rep 16:583–586
Paek KY, Chakrabarty D (2003) Micropropagation of woody plants using bioreactor. In: Jain SM, Ishii K (eds) Micropropagation of woody trees and fruits. Kluwer Academic Publishers, Dordrecht, pp 756–756
Paek KY, Hahn EJ, Son SH (2001) Application of bioreactors of large scale micropropagation systems of plants. In vitro Cell Dev Biol-Plant 37:149–157
Park SY, Huh YS, Paek KY (2018) Common protocol in orchid micropropagation. In: Lee YI, Yeung EC (eds) Orchid propagation: from laboratories to greenhouses-methods and protocols. Springer, New York, pp 179–193
Park SY, Murthy HN, Paek KY (2000) Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Organ Cult 63:67–72
Park SY, Murthy HN, Paek KY (2003) Protocorm-like body induction and subsequent plant regeneration from root tip cultures of Doritaenopsis. Plant Sci 164:919–923
Puhan P, Rath SP (2012) In vitro propagation of Aegle marmelos (L.) corr., a medicinal plant through axillary bud multiplication. Adv Biosci Biotechnol 3:121–125
Roy J, Banerjee N (2003) Induction of callus and plant regeneration from shoot-tip explants of Dendrobium fimbriatum Lindl. Var. oculatum Hk. F. Sci Hortic 97:333–340
Sajc L, Grubisic D, Novakovic GV (2000) Bioreactors for plant engineering: an out for further research. Biochem Eng J 4:89–99
Schuster R, Zeindl L, Holzer W, Khumpirapang N, Okonogi S, Viernstein H, Mueller M (2017) Eulophia macrobulbon – an orchid with significant anti-inflammatory and antioxidant effect and anticancerogenic potential exerted by its root extract. Phytomedicine 24:157–165
Seibert M, Wetherbee PJ, Job Đ (1975) The effects of light intensity and spectral quality on growth and shoot initiation in tobacco callus. Plant Physiol 56:130–139
Seok PR, Kim JH, Kwon HR, Heo JS, Choi JR, Shin JH (2018) Protective effects of Gastrodia elata Blume on acetaminophen-induced liver and kidney toxicity in rats. Food Sci Biotechnol 27:1445–1454
Sheelavantmath SS, Murthy HN, Pyati AN, Ashok Kumar HG, Ravishankar BV (2000) In vitro propagation of the endangered orchid, Geodorum densiflorum (lam.) Schltr. Through rhizome section culture. Plant Cell Tissue Organ Cult 60:151–154
Shiau YJ, Sagare AP, Chen UC, Yang SR, Tasy HS (2002) Conservation of Anoectochilus formosanus Hayata by artifical cross-pollination and in vitro culture of seeds. Bot Bull Acad Sin 43:123–130
Singh S, Singh AK, Kumar S, Kumar M, Pandey PK, Singh MCK (2012) Medicinal properties and uses of orchids: a concise review. Elixir Appl Bot 52:11627–11634
Son SH, Choi SM, Kwon SR, Lee YH, Paek KY (1999) Large scale culture of plant cell and tissue by bioreactor system. J Plant Biotech 1:1–8
Takayama S, Misawa MA (1981) Scheme for mass propagation of Lilium in vitro. Physiol Plant 48:121–125
Teixeira da Silva JA, Ng TB (2017) The medicinal and pharmaceutical importance of Dendrobium species. Appl Microbiol Biotechnol 101:2227–2239
Tomiczak K, Mikula A, Sliwinska E, Rybczyński JJ (2015) Autotetraploid plant regeneration by indirect somatic embryogenesis from leaf mesophyll protoplasts of diploid Gentianadecumbens L.f. In Vitro Cell Dev Biol Plant 51:250–359
Wang SY, Kuo YH, Chang HN, Kang PL, Tsay HS, Lin KF, Yang NS, Shyur LF (2002) Profiling and characterization of antioxidant activities in Anoectochilus formosanus Hayata. J Agric Food Chem 50:1859–1865
Wang HQ, Jin MY, Paek KY, Piao XC, Lian ML (2016) An efficient strategy for enhancement of bioactive compounds by protocorm-like body culture of Dendrobium candidum. Ind Crop Prod 84:121–130
Webb KJ, Street HE (1977) Morphogenesis in vitro of Pinus and Picea. Acta Hortic 78:259–269
Wei Q, Cao J, Qian W, Xu M, Li Z, Ding Y (2015) Establishment of an efficient micropropagation and callus regeneration system from the axillary buds of Bambusa ventricosa. Plant Cell Tissue Organ Cult 122:1–8
William DS, Gangaprasad A, Seeni S, Sarojini MV (2003) Micropropagation and ecorestoration of Vanda spathulata an exquisite orchid. Plant Cell Tiss Organ Cult 72:199–202
Wu ZH, Chakrabarty D, Hahn EJ, Paek KY (2007) Micropropagation of an endangered jewel orchid (Anoectochiuls formosanus) using bioreactor system. Hortic Environ Biotechnol 48:376–380
Yang F, Wei NN, Gao R, Piao XC, Lian ML (2015) Effect of several medium factors on polysaccharide and alkaloid accumulation in protocorm-like bodies of Dendrobium candidum during bioreactor culture. Acta Physiol Plant 37:94
Yoon YJ, Murthy HN, Hahn EJ, Paek KY (2007) Biomass production of Anoectochilus formosanus Hayata in a bioreactor system. J Plant Biol 50:573–576
Zhan HD, Zhou HY, Sui YP, Du XL, Wang WH, Dai L, Sui F, Huo HR, Jiang TL (2016) The rhizome of Gastrodia elata Blume – An ethnopharmacological review. J Ethnopharmacol 189:361–385
Zhao Y (2014) Auxin biosynthesis. The Arabidopsis Book 12:e0173. https://doi.org/10.1199/tab.0173
Zhao P, Wu F, Feng FS, Wang WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall exLindl. In Vitro Cell Dev Biol Plant 44:178
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Park, SY., Ho, TT., Paek, KY. (2020). Medicinal Orchids: Production of Bioactive Compounds and Biomass. In: Khasim, S., Hegde, S., González-Arnao, M., Thammasiri, K. (eds) Orchid Biology: Recent Trends & Challenges . Springer, Singapore. https://doi.org/10.1007/978-981-32-9456-1_22
Download citation
DOI: https://doi.org/10.1007/978-981-32-9456-1_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9455-4
Online ISBN: 978-981-32-9456-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)