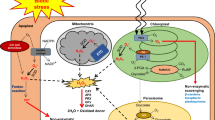
Abstract
Due to their immobility both in vitro and in vivo, crop plants are constantly exposed to abiotic and biotic factors. As a result, they have more sophisticated immune defences than animals. They might experience the mixture of these stressors concurrently or successively. The study of hydrogen peroxide (H2O2) is becoming more popular in the realm of molecular biology. It is a significant redox (reduction-oxidation reaction) metabolite that causes oxidative injury to biomolecules at high quantities, which can lead to cell death. Conversely, at low concentrations, H2O2 functions as a signalling molecule and mimics plant hormones in several ways. The hazardous nature of hydrogen peroxide was first understood to result in cell viability losses due to injury at several levels of cell organisation. It is now well-known that H2O2 has a positive role as a major hub integrating signalling network in response to abiotic stress and during developmental processes. In this chapter, the production, scavenging and the dual role of hydrogen peroxide from the point of view of its role in plant growth and developmental process and in abiotic stress tolerance have been presented.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Al-Saikhan MS, Shalaby TA (2019) Effect of hydrogen peroxide (H2O2) treatment on physicochemical characteristics of tomato fruits during post-harvest storage. Aust J Crop Sci 13:798–802
An Y, Feng X, Liu L, Xiong L, Wang L (2016) ALA-induced flavonols accumulation in guard cells is involved in scavenging H2O2 and inhibiting stomatal closure in Arabidopsis cotyledons. Front Plant Sci 7:1713
Antunes WC, de Menezes DD, Pinheiro DP, Williams TCR, Loureiro ME (2017) Guard cell-specific down-regulation of the sucrose transporter SUT1 leads to improved water use efficiency and reveals the interplay between carbohydrate metabolism and Kþ accumulation in the regulation of stomatal opening. Environ Exp Bot 135:73–85
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Ashfaque F, Khan MI, Khan NA (2014) Exogenously applied H2O2 promotes proline accumulation. Water relations. Photosynthetic efficiency and growth of wheat (Triticum aestivum L.) under salt stress. Annu Res Rev Biol 105e:20
Azevedo-Neto AD, Prisco JT, Eneas-Filho J, Medeiros JVR, Filho E (2005) Hydrogen peroxide pre-treatment induces salt stress acclimation in maize plants. J Plant Physiol 162:1114–1122
Bagheri M, Gholami M, Baninasab B (2019) Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Sci Hort 243:207e213
Banerjee A, Roychoudhury A (2018) Abiotic stress, generation of reactive oxygen species, and their consequences: An overview. In: Singh VP, Singh S, Tripathi DK, Prasad SM, Chauhan DK (eds) Reactive oxygen species in plants: boon or bane-revisiting the role of ROS, 1st edn. Wiley, pp 23–50
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, López-Jaramillo J, Padilla MN, Carreras A, Corpas FJ, Barroso BJ (2013) Dual regulation of cytosolic ascorbate peroxidase (APX)by tyrosine nitration and S-nitrosylation. J Exp Bot 65:527–538
Ben Rejeb K, Lefebvre-De Vos D, Le Disquet I, Leprince AS, Bordenave M, Maldiney R, Jdey A, Abdelly C, Savouré A (2015) Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitols tress in Arabidopsis thaliana. New Phytol 208:1138–1148
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65(5):1241–1257
Brewer TF, Garcia FJ, Onak CS, Carroll KS, Chang CJ (2015) Chemical approaches to discovery and study of source sand targets of hydrogen peroxide redox signalling through NAPDH oxidase proteins. Annu Rev Biochem 84:765–790
Brychkova G, Yarmolinsky D, Fluhr R, Sagi M (2012) The determination of sulphite levels and its oxidation in plant leaves. Plant Sci 190:123–130
Cerny M, Habanova H, Berka M, Luklova M, Brzobohaty B (2018) Hydrogen peroxide, its role in plant biology and crosstalk with signalling networks. Int J Mol Sci 19:2812
Chang Q, Tang H (2014) Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim Acta 181:527–534
Chao Y-Y, Hsu YT, Kao CH (2009) Involvement of glutathione in heat shock-and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 318:37–45
Chawla S, Goyal SC, Angrish R, Rani C, Arora V, Datta KS, Madaan S, Devi S (2010) Acclimatory response to hydrogen peroxide and glutathione under salt boron stress through their impact on mineral nutrition and antioxidant defense system in pigeonpea (Cajanus cajan L.). Physiol Mol Biol Plants 16:295e304
Cona A, Rea G, Botta M, Corelli F, Federico R, Angelini R (2006) Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J Exp Bot 57:2277–2289
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
De Carvalho MHC (2013) Drought stress and reactive oxygen species. Production, scavenging and signalling. Plant Signal Behav 3:156–165
Deng XP, Cheng YJ, Wu XB, Kwak SS, Chen W, Eneji AE (2012) Exogenous hydrogen peroxide positively influences root growth and exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Aust J Crop Sci 6:1572
Dickinson BC, Chang CJ (2011) Chemistry and bbiology of reactive oxygen species in signalling or stress responses. Nat Chem Biol 7:504–511
Dietz KJ, Mittler R, Noctor G (2016) Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol 171:1535–1539
Dikilitas M, Karakas S, Ahmad P (2018) Predisposition of crop plants to stress is directly related to their DNA health. In: Egamberdieva D, Ahmad P (eds) Plant microbiome: stress response. Springer, Singapore, pp 233–254
Fan Y, Huang Y (2012) The effective peroxidase-like activity of chitosan-functionalized CoFe2O4 nanoparticles for chemiluminescence sensing of hydrogen peroxide and glucose. Analyst 137(5):1225–1231
Fariduddin Q, Khan TA, Yusuf M (2014) Hydrogen peroxide mediated tolerance to copper stress in the presence of 28-homobrassinolide in Vigna radiata. Acta Physiol Plant 36(10):2767–2778
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119: 355–364
Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C (2015) Roles of cell wall peroxidases in plant development. Phytochemistry 112:15–21
Geros H, Chaves M, Delrot S (2012) The biochemistry of the grape fruit: USA: Bentham Science Publishers. ISBN 9781608053605, 304
Gondim FA, Gomes-Filho E, Costa JH, Mendes Alencar NLM, Prisco JT (2012) Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol Biochem 56:62–71
Gondim FA, Miranda RS, Gomes-Filho E, Prisco JT (2013) Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor Exp Plant Physiol 25:251–260
Gong M, Chen B, Li ZG, Guo LH (2001) Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J Plant Physiol 158:112–1130-00327
Grivennikova VG, Vinogradov AD (2013) Partitioning of superoxide and hydrogen peroxide production by mitochondrial respiratory complex I. Biochim Biophys Acta 1827:446–454
Guzel S, Terzi R (2013) Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot Stud 54:26
Hameed AM, Farooq SH, Iqbal NA, Arshad RU (2004) Influence of exogenous application of hydrogen peroxide on root and seedling growth on wheat (Triticum aestivum L.). Int J Agric Biol 6:366.e369
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L., an intrinsic study on antioxidant defense and glyoxalase systems Front Plant Sci 8:8.e115
He JM, Liu ZH, Xu H, She XP, Huang C (2006) The involvement of hydrogen peroxide in UV-B-inhibited pollen germination and tube growth of Paeonia suffruticosa and Paulownia tomentosa in vitro. Plant Growth Regul 49:199–208
Hossain MA, Fujita M (2013) Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.). Plant Gene & Trait 4:109–123
Hossain MA, Hossain AZ, Kihara T, Koyama H, Hara T (2005) Aluminum induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci Plant Nutr 51:223.e230
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycine betaine and proline in mungbean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Hossain MA, Mostofa MG, Fujita M (2013a) Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed 4:50–70
Hossain MA, Mostofa MG, Fujita M (2013b) Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Plant Sci Mol Breed 2:1–14
Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133:170–181
Iseri OD, Körpe DA, Sahin FI, Haberal M (2013) Hydrogen peroxide pretreatment of roots enhanced oxidative stress response of tomato under cold stress. Acta Physiol Plant 35:1905–1913-1228-7
Ishibashi Y, Yamaguchi H, Yuasa T, Iwaya-Inoue M, Arima S, Zheng SH (2011) Hydrogen peroxide spraying alleviates drought stress in soybean plants. J Plant Physiol 168:1562–1567
Jahan AA, Anis M (2014) Changes in antioxidative enzymatic responses during acclimatization of in vitro raised plantlets of Cardiospermum halicacabum L. against oxidative stress. J Plant Physiol Pathol 4:2
Jing LZ, Kui GY, Hang LS, Gang BJ (2009) Effects of exogenous hydrogen peroxide on ultrastructure of chloroplasts and activities of antioxidant enzymes in greenhouse-ecotype cucumber under drought stress. Acta Hortic Sin 36:1140–1146
Kang NJ, Kang YI, Kang KH, Jeong BR (2009) Induction of thermotolerance and activation of antioxidant enzymes in H2O2 pre-applied leaves of cucumber and tomato seedlings. J Jpn Soc Hortic Sci 78:320–329
Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R (2015) Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci 3:13
Khan MI, Khan NA, Masood A, Per TS, Asgher M (2016) Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use efficiency of nitrogen and sulfur, and glutathione production in mustard. Front Plant Sci 7:44
Khan TA, Yusuf M, Fariduddin Q (2018) Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress. Photosynthetica 56:1237–1248
Khandaker MM, Boyce AN, Osman N (2012) The influence of hydrogen peroxide on the growth, development and quality of wax apple (Syzygium samarangense, [Blume] Merrill & L.M. Perry var. jambu madu) fruits [Syzygium samarangense [Blume] Merrill & LM Perry var. jambu madu]. Plant Physiol Biochem 53:101–110
Kocyigit A, Guler EM, Dikilitas M (2017) Role of antioxidant phytochemicals in prevention, formation and treatment of cancer. In: Cristiana F, Elena A (eds) Reactive oxygen species (ROS) in living cells. Intech Open, pp 21–45
Krifka S, Hiller KA, Spagnuolo G, Jewett A, Schmalz G, Schweikl H (2012) The influence of glutathione on redox regulation by antioxidant proteins and apoptosis in macrophages exposed to 2-hydroxyethyl methacrylate (HEMA). Biomaterials 33:5177–5186
Kumari S, Verma VK (2019) Hydrogen peroxide mediates suberization, root thickness and stomatal movement in wheats. Int J Curr Microbiol App Sci 8:2448–2454
Li XP, Xu QQ, Liao WB, Ma ZJ, Xu XT, Wang M, Ren PJ, Niu LJ, Jin X, Zhu YC (2016) Hydrogen peroxide is involved in abscisic acid-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J Plant Biol 59:536–548
Liu ZJ, Guo YK, Bai JG (2010) Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. J Plant Growth Regul 29(2):171–183
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules 23(3):535
Mehler AH (1951) Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys 33:339–351 90082–3, 65
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by upregulating antioxidative and glyoxalase systems. Ecotoxicology 22:959–973. https://doi.org/10.1007/s10646-013-1073-x
Mostofa MG, Seraj ZI, Fujita M (2014a) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386
Mostofa MG, Yoshida N, Fujita M (2014b) Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul 73:31–44
Munne-Bosch S, Pinto-Marijuan M (2017) Free radicals, oxidative stress and antioxidants. Encycl Appl Plant Sci 2:16–19
Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195
Nazir F, Hussain A, Fariduddin Q (2019) Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 230:544–558
Nazir F, Fariduddin Q, Khan TA (2020) Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252:126486
Niu L, Liao W (2016) Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front Plant Sci 7:230
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol 80:3–12. https://doi.org/10.1016/j.semcdb.2017.07.013
Nyathi Y, Baker A (2006) Plant peroxisomes as a source of signalling molecules. Biochim Biophys Acta 1763:1478–1495
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants 2012:pls014
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994a) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen-peroxide. Plant Cell 6:65–74
Prasad TK, Anderson MD, Stewart CR (1994b) Acclimation, hydrogen peroxide, and abscisic-acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 10:619–627
Rani V, Deep G, Singh RK, Palle K, Yadav UC (2016) Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci 148:183–193
Remans T, Opdenakker K, Smeets K, Mathijsen D, Vangronsveld J, Cuypers A (2010) Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct Plant Biol 37:532–544
Sun Y, Zhang C, Qi A, Luo W (2009) Effects of hydrogen peroxide on the germination characteristics of cucumber seeds. In: International conference on environmental science and information application technology, pp 272.e275
Sun Y, Wang H, Liu S, Peng X (2016) Exogenous application of hydrogen peroxide alleviates drought stress in cucumber seedlings. S Afr J Bot 106:23–28
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523. https://doi.org/10.1016/S0168-9452(02)00159-0
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wang P, Du Y, Li Y, Ren D, Song CP (2010a) Hydrogen peroxide mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22:2981–2998
Wang Y, Li J, Wang J, Li Z (2010b) Exogenous H2O2 improves the chilling tolerance of manila grass and Mascarene grass by activating the antioxidative system. Plant Growth Regul 61:195–204
Wang Y, Zhang J, Li JL, Ma XR (2014) Exogenous hydrogen peroxide enhanced the thermotolerance of Festuca arundinacea and Lolium perenne by increasing the antioxidative capacity. Acta Physiol Plant 36:2915–2924
Wen JF, Gong M, Liu Y, Hu JL, Deng MH (2013) Effect of hydrogen peroxide on growth and activity of some enzymes involved in proline metabolism of sweet corn seedlings under copper stress. Sci Hortic (Canterb) 164:366e371
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence inC3 plants. EMBO J 16:4806–4816
Wu Z, Zhang C, Yan J, Yue Q, Ge Y (2015) Effects of sulfur supply and hydrogen peroxide pretreatment on the responses by rice under cadmium stress. Plant Growth Regul 77:299–306
Xiong J, Yang Y, Fu G, Tao L (2015) Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and dimethyl esterification in the cell wall of rice (Oryza sativa) root tips. New Phytol 206:118.e126
Xu PL, Guo YK, Bai JG, Shang L, Wang XJ (2008) Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivar sunder lowlight. Physiol Plant 132:467–478
Xu FJ, Jin CW, Liu WJ (2010) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. Plant Biol 54:44–53
Yıldız M, Terzi H, Bingül N (2013) Protective role of hydrogen peroxide pretreatment on defense systems and BnMP1 gene expression in Cr(VI)-stressed canola seedlings. Ecotoxicology 22:1303–1312
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Zhang YH, She XP (2012) The involvement of nitric oxide and hydrogen peroxide in eATP-inhibited pollen germination and tube growth of Paulownia tomentosa in vitro. Adv Mater Res 343:347.e350
Zhang H, Lv S, Xu H, Hou D, Li Y, Wang F (2017) H2O2 is involved in the metallothionein-mediated rice tolerance to copper and cadmium toxicity. Int J Mol Sci 18:2083
Zhou J, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ (2014) OH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J Exp Bot 65:595.e607
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jahan, A., Khan, M.M.A., Ahmad, B., Ahmed, K.B.M., Pandey, R.P., Gulfishan, M. (2023). Hydrogen Peroxide: Regulator of Plant Development and Abiotic Stress Response. In: Faizan, M., Hayat, S., Ahmed, S.M. (eds) Reactive Oxygen Species. Springer, Singapore. https://doi.org/10.1007/978-981-19-9794-5_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-9794-5_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9793-8
Online ISBN: 978-981-19-9794-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)