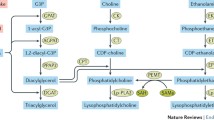
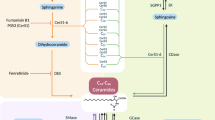
Abstract
Obesity research has shifted in recent years to address not only the total amount of adipose tissue present in an individual but also to include adipose tissue functions such as endocrine function and thermogenesis. Data suggest that sphingolipids are critical regulators of metabolic homeostasis, and that disruption of their levels is associated with metabolic disease. Abundant data from mouse models has revealed both beneficial and deleterious roles for sphingolipids in adipose function, and numerous human studies have shown that obesity alters circulating sphingolipid profiles. Sphingolipids comprise a large family of interrelated metabolites, and pinpointing specific functions for specific lipids will be required to fully exploit the therapeutic potential of targeting sphingolipids to treat obesity and related disorders.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hengst, J. A., Francy-Guilford, J. M., Fox, T. E., Wang, X., Conroy, E. J., & Yun, J. K. (2009). Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Archives of Biochemistry and Biophysics, 492(1–2), 62. https://doi.org/10.1016/J.ABB.2009.09.013
Takabe, K., Paugh, S. W., Milstien, S., & Spiegel, S. (2008). Inside-out signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacological Reviews. https://doi.org/10.1124/pr.107.07113
Wattenberg, B. W. (2010). Role of sphingosine kinase localization in sphingolipid signaling. World Journal of Biological Chemistry, 1(12), 362–368. https://doi.org/10.4331/wjbc.v1.i12.362
Maceyka, M., Harikumar, K. B., Milstien, S., & Spiegel, S. (2012). Sphingosine-1-phosphate signaling and its role in disease. Trends in Cell Biology. https://doi.org/10.1016/j.tcb.2011.09.003
Moseti, D., Regassa, A., & Kim, W. K. (2016). Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. International Journal of Molecular Sciences, 17(1), 124. https://doi.org/10.3390/IJMS17010124
Sarjeant, K., & Stephens, J. M. (2012). Adipogenesis. Cold Spring Harbor Perspectives in Biology, 4(9), 8417. https://doi.org/10.1101/CSHPERSPECT.A008417
Barbarroja, N., Rodriguez-Cuenca, S., Nygren, H., Camargo, A., Pirraco, A., Relat, J., Cuadrado, I., et al. (2015). Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes, 64(4), 1180–1192. https://doi.org/10.2337/DB14-0359
Wu, X., Sakharkar, M. K., Wabitsch, M., Yang, J., Sakharkar, M. K., Wabitsch, M., & Yang, J. (2020). Effects of sphingosine-1-phosphate on cell viability, differentiation, and gene expression of adipocytes. International Journal of Molecular Sciences, 21, 9284. https://doi.org/10.3390/ijms21239284
Wang, J., Badeanlou, L., Bielawski, J., Ciaraldi, T. P., & Samad, F. (2014). Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance. The American Journal of Physiology - Endocrinology and Metabolism, 306(7), 756–768. https://doi.org/10.1152/ajpendo.00549.2013
Anderson, A. K., Lambert, J. M., Montefusco, D. J., Tran, B. N., Roddy, P., Holland, W. L., & Ashley Cowart, L. (2020). Depletion of adipocyte sphingosine kinase 1 leads to cell hypertrophy, impaired lipolysis, and nonalcoholic fatty liver disease. Journal of Lipid Research, 61(10), 1328–1340. https://doi.org/10.1194/jlr.RA120000875
Ravichandran, S., Finlin, B. S., Kern, P. A., & Özcan, S. (2019). Sphk2−/− mice are protected from obesity and insulin resistance. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1865(3), 570–576. https://doi.org/10.1016/j.bbadis.2018.12.012
Chaurasia, B., Kaddai, V. A., Lancaster, G. I., Henstridge, D. C., Sriram, S., Galam, D. L. A., Gopalan, V., et al. (2016). Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metabolism, 24(6), 820–834. https://doi.org/10.1016/j.cmet.2016.10.002
Chaurasia, B., Tippetts, T. S., Mayoral Monibas, R., Liu, J., Li, Y., Wang, L., Wilkerson, J. L., et al. (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science, 365(6451), 386–392. https://doi.org/10.1126/SCIENCE.AAV3722
Chaurasia, B., Ying, L., Talbot, C. L., Maschek, J. A., Cox, J., Schuchman, E. H., Hirabayashi, Y., Holland, W. L., & Summers, S. A. (2021). Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes. Molecular Metabolism, 45, 101145. https://doi.org/10.1016/j.molmet.2020.101145
Ussher, J. R., Timothy, R., Koves, V. J., Cadete, J., Zhang, L., Jaswal, J. S., Swyrd, S. J., Lopaschuk, D. G., et al. (2010). Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. https://doi.org/10.2337/db09
Yang, G., Badeanlou, L., Bielawski, J., Roberts, A. J., Hannun, Y. A., & Samad, F. (2009). Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. American Journal of Physiology. Endocrinology and Metabolism, 297(1), 2008. https://doi.org/10.1152/AJPENDO.91014.2008
Sprangers, B., Pirenne, J., van Etten, E., Mark Waer, C., Mathieu, A., & Billiau, D. (2008). Other forms of immunosuppression. Kidney Transplantation, 6, 333–349. https://doi.org/10.1016/B978-1-4160-3343-1.50025-6
Goedecke, J. H., Gibson, A. S. C., Grobler, L., Collins, M., Noakes, T. D., & Lambert, E. V. (2000). Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes. American Journal of Physiology - Endocrinology and Metabolism, 279(6), 1325–1334. https://doi.org/10.1152/AJPENDO.2000.279.6.E1325/ASSET/IMAGES/LARGE/H11200203002.JPEG
Holland, W. L., Brozinick, J. T., Wang, L. P., Hawkins, E. D., Sargent, K. M., Liu, Y., Narra, K., et al. (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism, 5(3), 167–179. https://doi.org/10.1016/J.CMET.2007.01.002
Anthonsen, M. W., Rönnstrand, L., Wernstedt, C., Degerman, E., & Holm, C. (1998). Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. Journal of Biological Chemistry, 273(1), 215–221. https://doi.org/10.1074/JBC.273.1.215
Watt, M. J., Holmes, A. G., Pinnamaneni, S. K., Garnham, A. P., Steinberg, G. R., Kemp, B. E., & Febbraio, M. A. (2006). Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. American Journal of Physiology - Endocrinology and Metabolism, 290(3), 500–508. https://doi.org/10.1152/AJPENDO.00361.2005/ASSET/IMAGES/LARGE/ZH10030644430004.JPEG
Vroegrijk, I. O. C. M., Van Klinken, J. B., Van Diepen, J. A., Van Den Berg, S. A. A., Febbraio, M., Steinbusch, L. K. M., Glatz, J. F. C., et al. (2013). CD36 is important for adipocyte recruitment and affects lipolysis. Obesity, 21, 2037–2045. https://doi.org/10.1002/oby.20354
Funcke, J. B., & Scherer, P. E. (2019). Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. Journal of Lipid Research, 60(10), 1648–1697. https://doi.org/10.1194/jlr.R094060
Romacho, T., Elsen, M., Röhrborn, D., & Eckel, J. (2014). Adipose tissue and its role in organ crosstalk. Acta Physiologica (Oxford, England), 210(4), 733–753. https://doi.org/10.1111/APHA.12246
Zhang, W., Mottillo, E. P., Zhao, J., Gartung, A., VanHecke, G. C., Lee, J. F., Maddipati, K. R., et al. (2014). Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. Journal of Biological Chemistry, 289(46), 32178–32185. https://doi.org/10.1074/jbc.M114.601096
Samad, F., Hester, K. D., Yang, G., Hannun, Y. A., & Bielawski, J. (2006). Altered adipose and plasma sphingolipid metabolism in obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes, 55(9), 2579–2587. https://doi.org/10.2337/DB06-0330
Gohlke, S., Zagoriy, V., Inostroza, A. C., Méret, M., Mancini, C., Japtok, L., Schumacher, F., et al. (2019). Identification of functional lipid metabolism biomarkers of brown adipose tissue aging. Molecular Metabolism, 24(June), 1–17. https://doi.org/10.1016/j.molmet.2019.03.011
Fisher, F., Folliott, F., Kleiner, S., Douris, N., Fox, E. C., Mepani, R. J., Verdeguer, F., Jun, W., et al. (2012). FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes and Development. https://doi.org/10.1101/gad.177857.111
Fischer, A. W., Cannon, B., & Nedergaard, J. (2018). Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Molecular Metabolism, 7(January), 161–170. https://doi.org/10.1016/j.molmet.2017.10.009
Lodhi, I. J., & Semenkovich, C. F. (2009). Why we should put clothes on mice. Cell Metabolism. https://doi.org/10.1016/j.cmet.2009.01.004
Christoffersen, C., Federspiel, C. K., Borup, A., Holst, B., Heeren, J., & Nielsen, L. B. (2018). The apolipoprotein M/S1P axis controls triglyceride metabolism and brown fat activity. Cell Reports, 22, 175–188. https://doi.org/10.1016/j.celrep.2017.12.029
Karuna, R., Park, R., Othman, A., Holleboom, A. G., Motazacker, M. M., Sutter, I., Kuivenhoven, J. A., et al. (2011). Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis, 219(2), 855–863. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2011.08.049
Blachnio-Zabielska, A. U., Koutsari, C., Tchkonia, T., & Jensen, M. D. (2012). Sphingolipid content of human adipose tissue: Relationship to adiponectin and insulin resistance. Obesity, 20(12), 2341–2347. https://doi.org/10.1038/OBY.2012.126
Błachnio-Zabielska, A. U., Pułka, M., Baranowski, M., Nikołajuk, A., Zabielski, P., Górska, M., & Górski, J. (2012). Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. Journal of Cellular Physiology, 227(2), 550–557. https://doi.org/10.1002/JCP.22745
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Valentine, Y., Cowart, L.A. (2022). Sphingolipids in Adipose: Kin or Foe?. In: Jiang, XC. (eds) Sphingolipid Metabolism and Metabolic Disease. Advances in Experimental Medicine and Biology, vol 1372. Springer, Singapore. https://doi.org/10.1007/978-981-19-0394-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-0394-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0393-9
Online ISBN: 978-981-19-0394-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)