Abstract
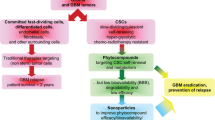
Every year, large populations of primary malignant brain tumors are reported, of which approximately 70% are malignant gliomas. Over half of malignant glioma patients are of glioblastoma multiforme (GBM) linked with high mortality rates. The progression of GBM leads through several complicated pathways, which have also been expressed in the recurring segment. Previous researches have shown that various compounds found in edible plants, known colloquially as phytochemicals, can simultaneously influence multiple genetic pathways and can be used to treat GBM as a potential drug agent. Polysaccharides and flavonoids are among the phytochemicals extensively analyzed for their antioxidant, antineoplastic, and anti-inflammatory actions. Likewise, a broad detail of phytochemicals that have significant effects on GBM had been provided. Green nanoparticles have the ability to prevent or reverse carcinogenesis by halting or redirecting the start process or stopping the progression phase. Centered on a deep understanding of the intrinsic properties of GBM, such novel methods of drug delivery have demonstrated promise to overcome certain barriers. The BBB obstructs drug distribution to the brain and inhibits the efficacy of both old and new medications at the specified location. Because current GBM treatments are preventative instead of therapeutic, new delivery mechanisms are critical, and nanoparticles will be at the forefront of future initiatives. This chapter discusses the role of natural phytochemicals in enhancing glioblastoma patient expectancy and life expectancy by increasing treatment potential and reducing significant side effects. Furthermore, many novel GBM treatments will use better delivery systems and abandon the current approach of injecting medications and devices directly into the tumor. Nano-biotechnology, especially nanoparticles, contributes significantly to improving the delivery of drugs into carcinoma cells, and many of these technologies can be used in GBM. Finally, this chapter therefore emphasized the potential of natural products and novel drug delivery systems in GBM treatment by regulating multiple cancer pathways, such as toxicity reduction and side effects.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- GBM:
-
Glioblastoma multiforme
- WHO:
-
World health organization
- PDGF:
-
Platelet-derived growth factor
- MDM2:
-
Mouse double minute 2
- EGFR:
-
Epidermal growth factor receptor
- PTEN:
-
Phosphatase and tensin homolog
- Wnt:
-
Wingless-related integration site
- PI3K:
-
Phosphatidylinositol 3-kinase
- AKT/PKB:
-
Protein kinase B
- LRP5:
-
Low-density lipoprotein receptor-related protein 5
- MMPs:
-
Matrix metalloproteinases
- Fz:
-
Frizzled receptor
- P56:
-
Transcription factor
- IKK:
-
IκB kinase
- Nf-kB:
-
Nuclear factor kappa B
- ICAM-1:
-
Intercellular adhesion molecule-1
- Bcl-2:
-
B cell lymphoma
- INK4:
-
Cyclin-dependent kinase inhibitors
- IL-8:
-
Interleukin
- VEGF:
-
Vascular endothelial growth factor
- MAPK:
-
mitogen-activated protein kinase
- RelA:
-
Rel Avian Reticuloendotheliosis Viral Oncogene Homolog A
- TGF-α:
-
Transforming growth factor-α
- Ser473 and Thr308:
-
serine/threonine kinase sites
- mTOR:
-
The mechanistic target of rapamycin
- TCS1/2:
-
Tuberous sclerosis complex
- PTCH:
-
Transmembrane receptor Patched
- GLI1:
-
Glioma-associated oncogene
- PTCH1:
-
Protein patched homolog 1
- PDGF-R:
-
Platelet-derived growth factor receptors
- QCT:
-
Quercetin
- STAT3:
-
Signal transducer and activator of transcription 3
- RVT:
-
Resveratrol
- BBB:
-
Blood-brain barrier
- ROS:
-
Reactive oxygen species
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- DMBA:
-
Dimethylbenz(a)anthracene
- UPA/UPAR:
-
Urokinase receptor
- TNF-α:
-
Tumor Necrosis Factor Alpha
- CCM:
-
Curcumin
- BAD:
-
BCL2 Associated Agonist of Cell Death
- COX-2:
-
Cyclooxygenase-2
- CRS:
-
Chrysin
- PPAR:
-
Peroxisome proliferator-activated receptor
- ERK:
-
a type of serine/threonine protein kinase
- TBK1 :
-
TANK-binding kinase 1
- MCF-7:
-
Michigan Cancer Foundation-7
- NRF-2:
-
Nuclear factor erythroid 2-related factor 2
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- GST:
-
Genistein
- BC-A:
-
Biochanin A
- EGG:
-
Epigallocatechin gallate
- HeLa:
-
Henrietta Lacks
- LNCaP:
-
Lymph Node Carcinoma
- PARP:
-
Poly (ADP-ribose) polymerase
- CDK:
-
Cyclin-dependent kinase
- CD:
-
Cluster of Differentiation
- LTN:
-
Lentinan
- Dectin-1:
-
C-type lectin domain family 7 member A
- CR3:
-
Complement receptors 3
- NK:
-
Natural killer cells
- TH1/2:
-
T helper
- DLD:
-
D.L. Dexter
- PD-L1:
-
Programmed cell death ligand
- RTN:
-
Retinoids
- OPBA:
-
Ophiobolin A
- ER stress:
-
Endoplasmic Reticulum Stress
- ITC:
-
Isothiocyanates
- TMZ:
-
Temozolomide
- ECM:
-
Extracellular matrix
- SKN:
-
Shikonin
- DRB:
-
Doxorubicin
- NPs:
-
Nanoparticles
- SPA:
-
Super-paramagnetic particle adducts
- GW:
-
Gliadel Wafers
- FDA:
-
Food and Drug Administration
- BM:
-
Biomimetic
- CLT:
-
Cyclic decapeptide
- mAb:
-
Monoclonal antibody
- PEBBLEs:
-
Probes encapsulated by biologically localized embedding
References
Kane JR. The role of brain vasculature in glioblastoma. Mol Neurobiol. 2019;56(9):6645–53.
Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7.
Wang HH, Chang TY, Lin WC, Wei KC, Shin JW. GADD45A plays a protective role against temozolomide treatment in glioblastoma cells. Sci Rep. 2017;7(1):1–5.
Laug D, Glasgow SM, Deneen B. A glial blueprint for gliomagenesis. Nat Rev Neurosci. 2018;19(7):393–403.
Desai V, Bhushan A. Natural bioactive compounds: alternative approach to the treatment of glioblastoma multiforme. Biomed Res Int. 2017;2017:9363040.
Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. 2008;7(9):1321–5.
Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5):S2.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells nature. Nature. 2004;432(7015):396–401.
Aoki T, Hashimoto N, Matsutani M. Management of glioblastoma. Expert Opin Pharmacother. 2007;8(18):3133–46.
Gallego JM, Barcia JA, Barcia-Marino C. Fatal outcome related to carmustine implants in glioblastoma multiforme. Acta Neurochir. 2007;149(3):261–5.
Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci. 2000;97(12):6803–8.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061.
Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Caeiro C. Current standards of care in glioblastoma therapy. Brisbane City, QLD: Exon Publications; 2017. p. 197–241.
Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol/Oncol Clin. 2012;26(4):825–53.
Koc K, Anik I, Cabuk BU, Ceylan SA. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg. 2008;22(1):99–103.
Vidak M, Rozman D, Komel R. Effects of flavonoids from food and dietary supplements on glial and glioblastoma multiforme cells. Molecules. 2015;20(10):19406–32.
Haque A, Banik NL, Ray SK. Molecular alterations in glioblastoma: potential targets for immunotherapy. Prog Mol Biol Transl Sci. 2011;98:187–234.
Atiq A, Parhar I. Anti-neoplastic potential of flavonoids and polysaccharide phytochemicals in glioblastoma. Molecules. 2020;25(21):4895.
Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3(3):198–210.
Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, Wu W, James CD, Sarkaria JN. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009;11(3):281–91.
Singhal N, Selva-Nayagam S, Brown MP. Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment-case study and review of literature. J Neurooncol. 2007;85(2):229–30.
Abbas MN, Kausar S, Cui H. Therapeutic potential of natural products in glioblastoma treatment: Targeting key glioblastoma signaling pathways and epigenetic alterations. Clin Transl Oncol. 2020;22(7):963–77.
Wang G, Wang J, Du L, Li F. Effect and mechanism of total flavonoids extracted from cotinus coggygria against glioblastoma cancer in vitro and in vivo. Biomed Res Int. 2015;2015:856349.
Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–80.
Nager M, Bhardwaj D, Cantí C, Medina L, Nogués P, Herreros J. β-Catenin signalling in glioblastoma multiforme and glioma-initiating cells. Chemother Res Pract. 2012;192362:2012.
Kaur N, Chettiar S, Rathod S, Rath P, Muzumdar D, Shaikh ML, Shiras A. Wnt3a mediated activation of Wnt/β-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci. 2013;54:44–57.
Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, Arita K. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011;102(3):540–8.
Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, Jiang H. Downregulation of Wnt2 and β-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16(4):351–61.
Onyido EK, Sweeney E, Nateri AS. Wnt-signalling pathways and microRNAs network in carcinogenesis: experimental and bioinformatics approaches. Mol Cancer. 2016;15(1):1–7.
Liu Y, Yan W, Zhang W, Chen L, You G, Bao Z, Wang Y, Wang H, Kang C, Jiang T. MiR-218 reverses high invasiveness of glioblastoma cells by targeting the oncogenic transcription factor LEF1. Oncol Rep. 2012;28(3):1013–21.
Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–46.
Park JB, Agnihotri S, Golbourn B, Bertrand KC, Luck A, Sabha N, Smith CA, Byron S, Zadeh G, Croul S, Berens M. Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget. 2014;5(19):9382.
Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362(1):1–7.
Weeks A, Okolowsky N, Golbourn B, Ivanchuk S, Smith C, Rutka JT. ECT2 and RASAL2 mediate mesenchymal-amoeboid transition in human astrocytoma cells. Am J Pathol. 2012;181(2):662–74.
Nager M, Bhardwaj D, Cantí C, Medina L, Nogués P, Herreros J. β-Catenin signalling in glioblastoma multiforme and glioma-initiating cells. Chemother Res Pract. 2012;2012:192362.
Gao X, Mi Y, Ma Y, Jin W. LEF1 regulates glioblastoma cell proliferation, migration, invasion, and cancer stem-like cell self-renewal. Tumor Biol. 2014;35(11):11505–11.
Guo G, Kuai D, Cai S, Xue N, Liu Y, Hao J, Fan Y, Jin J, Mao X, Liu B, Zhong C. Knockdown of FRAT1 expression by RNA interference inhibits human glioblastoma cell growth, migration and invasion. PLoS One. 2013;8(4):e61206.
Kita D, Yonekawa Y, Weller M, Ohgaki H. PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 2007;113(3):295–302.
Yamini B, Yu X, Dolan ME, Wu MH, Kufe DW, Weichselbaum RR. Inhibition of nuclear factor-κB activity by temozolomide involves O6-methylguanine–induced inhibition of p65 DNA binding. Cancer Res. 2007;67(14):6889–98.
Nagai S, Washiyama K, Kurimoto M, Takaku A, Endo S, Kumanishi T. Aberrant nuclear factor-κB activity and its participation in the growth of human malignant astrocytoma. J Neurosurg. 2002;96(5):909–17.
Galvani E, Sun J, Leon LG, Sciarrillo R, Narayan RS, Sjin RT, Lee K, Ohashi K, Heideman DA, Alfieri RR, Heynen GJ. NF-κB drives acquired resistance to a novel mutant-selective EGFR inhibitor. Oncotarget. 2015;6(40):42717.
Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-κB activation by inhibiting IκBα kinase activation, thereby suppressing NF-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280(17):17203–12.
Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2(9):823–30.
Atkinson GP, Nozell SE, Benveniste ET. NF-κB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 2010;10(4):575–86.
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, Wu J, Hu B, Cheng SY, Li M, Li J. TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J Clin Invest. 2012;122(10):3563–78.
Roshan MK, Soltani A, Soleimani A, Kahkhaie KR, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–34.
Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65(19):2979–99.
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3–induced NF-κB activation. Nat Immunol. 2004;5(5):503–7.
Festjens N, Berghe TV, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14(3):400–10.
Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P, Tan P, Sah DW, Cavenee WK, Furnari FB. EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway. Oncogene. 2012;31(36):4054–66.
Xie TX, Xia Z, Zhang NU, Gong W, Huang S. Constitutive NF-κB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23(3):725–32.
Shostak K, Chariot A. EGFR and NF-κB: partners in cancer. Trends Mol Med. 2015;21(6):385–93.
Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22(11):1490–500.
Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFκB and the essentialness of NFκB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125(12):2863–70.
Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-κB activation through Gab1 in glioblastoma cells. Mol Cell Biol. 2004;24(2):823–36.
Pearson JR, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Targeted Ther. 2017;2(1):1–1.
Puliyappadamba VT, Chakraborty S, Chauncey SS, Li L, Hatanpaa KJ, Mickey B, Noorani S, Shu HK, Burma S, Boothman DA, Habib AA. Opposing effect of EGFRWT on EGFRvIII-mediated NF-κB activation with RIP1 as a cell death switch. Cell Rep. 2013;4(4):764–75.
Robe PA, Bentires-Alj M, Bonif M, Rogister B, Deprez M, Haddada H, Khac MT, Jolois O, Erkmen K, Merville MP, Black PM. In vitro and in vivo activity of the nuclear factor-κB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10(16):5595–603.
Wieland A, Trageser D, Gogolok S, Reinartz R, Höfer H, Keller M, Leinhaas A, Schelle R, Normann S, Klaas L, Waha A. Anticancer effects of niclosamide in human glioblastoma. Clin Cancer Res. 2013;19(15):4124–36.
Pelloski CE, Lin E, Zhang L, Yung WA, Colman H, Liu JL, Woo SY, Heimberger AB, Suki D, Prados M, Chang S. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12(13):3935–41.
Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110(6):775–87.
Koul D, Fu J, Shen R, LaFortune TA, Wang S, Tiao N, Kim YW, Liu JL, Ramnarian D, Yuan Y, Garcia-Echevrria C. Antitumor activity of NVP-BKM120—a selective pan class I PI3 kinase inhibitor showed differential forms of cell death based on p53 status of glioma cells. Clin Cancer Res. 2012;18(1):184–95.
Pitz MW, Eisenhauer EA, MacNeil MV, Thiessen B, Easaw JC, Macdonald DR, Eisenstat DD, Kakumanu AS, Salim M, Chalchal H, Squire J. Phase II study of PX-866 in recurrent glioblastoma. Neuro Oncol. 2015;17(9):1270–4.
Ma DJ, Galanis E, Anderson SK, Schiff D, Kaufmann TJ, Peller PJ, Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17(9):1261–9.
Kubiatowski T, Jang T, Lachyankar MB, Salmonsen R, Nabi RR, Quesenberry PJ, Litofsky NS, Ross AH, Recht LD. Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J Neurosurg. 2001;95(3):480–8.
O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–8.
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–83.
Gulati N, Karsy M, Albert L, Murali R, Jhanwar-Uniyal M. Involvement of mTORC1 and mTORC2 in regulation of glioblastoma multiforme growth and motility. Int J Oncol. 2009;35(4):731–40.
Wu SH, Bi JF, Cloughesy T, Cavenee WK, Mischel PS. Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance. Cancer Biol Med. 2014;11(4):255.
Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804(3):433–9.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–45.
Fonović M, Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta. 2014;1840(8):2560–70.
Frame MC, Brunton VG. Advances in Rho-dependent actin regulation and oncogenic transformation. Curr Opin Genet Dev. 2002;12(1):36–43.
Clement V, Sanchez P, De Tribolet N, Radovanovic I, i Altaba AR. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–72.
Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancer. 2016;8(2):22.
Bigelow RL, Chari NS, Undén AB, Spurgers KB, Lee S, Roop DR, Toftgård R, McDonnell TJ. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279(2):1197–205.
Zhu H, Lo HW. The human glioma-associated oncogene homolog 1 (GLI1) family of transcription factors in gene regulation and diseases. Curr Genomics. 2010;11(4):238–45.
Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F. A role of PDGFRα in basal cell carcinoma proliferation. Proc Natl Acad Sci. 2001;98(16):9255–9.
Cao X, Geradts J, Dewhirst MW, Lo HW. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene. 2012;31(1):104–15.
Dikshit B, Irshad K, Madan E, Aggarwal N, Sarkar C, Chandra PS, Gupta DK, Chattopadhyay P, Sinha S, Chosdol K. FAT1 acts as an upstream regulator of oncogenic and inflammatory pathways, via PDCD4, in glioma cells. Oncogene. 2013;32(33):3798–808.
Lo HW, Zhu H, Cao X, Aldrich A, Ali-Osman F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69(17):6790–8.
Zhu H, Carpenter RL, Han W, Lo HW. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett. 2014;343(1):51–61.
Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–28.
Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12(8):930–3.
Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–13.
Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11(6):733–40.
Perez-Vizcaino F, Duarte J, Andriantsitohaina R. Endothelial function and cardiovascular disease: effects of quercetin and wine polyphenols. Free Radic Res. 2006;40(10):1054–65.
Chen SF, Nieh S, Jao SW, Liu CL, Wu CH, Chang YC, Yang CY, Lin YS. Quercetin suppresses drug-resistant spheres via the p38 MAPK–Hsp27 apoptotic pathway in oral cancer cells. PLoS One. 2012;7(11):e49275.
Russo M, Palumbo R, Tedesco I, Mazzarella G, Russo P, Iacomino G, Russo GL. Quercetin and anti-CD95 (Fas/Apo1) enhance apoptosis in HPB-ALL cell line. FEBS Lett. 1999;462(3):322–8.
Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318(8):925–35.
Sang DP, Li RJ, Lan Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014;35(6):832–8.
Kim H, Moon JY, Ahn KS, Cho SK. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496.
Zamin LL, Filippi-Chiela EC, Vargas J, Demartini DR, Meurer L, Souza AP, Bonorino C, Salbego C, Lenz G. Quercetin promotes glioma growth in a rat model. Food Chem Toxicol. 2014;63:205–11.
El-Readi MZ, Eid S, Abdelghany AA, Al-Amoudi HS, Efferth T, Wink M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine. 2019;55:269–81.
Vervandier-Fasseur D, Latruffe N. The potential use of resveratrol for cancer prevention. Molecules. 2019;24(24):4506.
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X, Wang S, Zhao J, Li Y, Cao Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients. 2015;7(6):4383–402.
Westhoff MA, Zhou S, Nonnenmacher L, Karpel-Massler G, Jennewein C, Schneider M, Halatsch ME, Carragher NO, Baumann B, Krause A, Simmet T. Inhibition of NF-κB signaling ablates the invasive phenotype of glioblastoma. Mol Cancer Res. 2013;11(12):1611–23.
Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol Rep. 2012;27(6):2050–6.
Li F, Sethi G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–80.
Hegi ME, Diserens AC, Gorlia T, Hamou MF, De Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Arepalli SK, Choi M, Jung JK, Lee H. Novel NF-κB inhibitors: a patent review (2011–2014). Expert Opin Ther Pat. 2015;25(3):319–34.
Sehdev V, Lai JC, Bhushan A. Biochanin A modulates cell viability, invasion, and growth promoting signaling pathways in HER-2-positive breast cancer cells. J Oncol. 2009;2009:121458.
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20.
Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67(15):1641–53.
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–9.
Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res. 2011;4(9):1419–25.
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–11.
Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27(1):40–8.
Ryu J, Ku BM, Lee YK, et al. Resveratrol reduces tnf-alpha-induced u373mg human glioma cell invasion through regulating nf-kappa b activation and upa/upar expression. Anticancer Res. 2011;31:4223–30.
Filippi-Chiela EC, Thomé MP. e Silva MM, Pelegrini AL, Ledur PF, Garicochea B, Zamin LL, Lenz G. Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells. BMC Cancer. 2013;13(1):1–3.
Cilibrasi C, Riva G, Romano G, Cadamuro M, Bazzoni R, Butta V, Paoletta L, Dalprà L, Strazzabosco M, Lavitrano M, Giovannoni R. Resveratrol impairs glioma stem cells proliferation and motility by modulating the wnt signaling pathway. PLoS One. 2017;12(1):e0169854.
Paul I, Bhattacharya S, Chatterjee A, Ghosh MK. Current understanding on EGFR and Wnt/β-catenin signaling in glioma and their possible crosstalk. Genes Cancer. 2013;4(11–12):427–46.
Götze S, Wolter M, Reifenberger G, Müller O, Sievers S. Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer. 2010;126(11):2584–93.
Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90.
Perry MC, Demeule M, Régina A, Moumdjian R, Béliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res. 2010;54(8):1192–201.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18.
Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131(5):682–91.
Ramachandran C, Resek AP, Escalon E, Aviram A, Melnick SJ. Potentiation of gemcitabine by Turmeric Force™ in pancreatic cancer cell lines. Oncol Rep. 2010;23(6):1529–35.
Ramachandran C, Nair SM, Escalon E, Melnick SJ. Potentiation of etoposide and temozolomide cytotoxicity by curcumin and turmeric force in brain tumor cell lines. J Complement Integr Med. 2012;9(1):Article 20.
Ji M, Choi J, Lee J, Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14(2):253–6.
Ramachandran C, Quirin KW, Escalon EA, Lollett IV, Melnick SJ. Therapeutic effect of supercritical CO2 extracts of Curcuma species with cancer drugs in rhabdomyosarcoma cell lines. Phytother Res. 2015;29(8):1152–60.
Ramachandran C, Portalatin G, Quirin KW, Escalon E, Khatib Z, Melnick SJ. Inhibition of AKT signaling by supercritical CO2 extract of mango ginger (Curcuma amada Roxb.) in human glioblastoma cells. J Complement Integr Med. 2015;12(4):307–15.
Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86.
Hanahan D, Weinberg RA. The hallmarks of cancer cell. 2000;100(1):57–70.
Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, Schäfer H. Role of NF-κ B and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–51.
Cheng JQ, Jiang X, Fraser M, Li M, Dan HC, Sun M, Tsang BK. Role of X-linked inhibitor of apoptosis protein in chemoresistance in ovarian cancer: possible involvement of the phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat. 2002;5(3–4):131–46.
Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 2010;16(12):1410–6.
Whang YE, Yuan XJ, Liu Y, Majumder S, Lewis TD. Regulation of sensitivity to TRAIL by the PTEN tumor suppressor. Vitam Horm. 2004;67:409–26.
Downward J. PI 3-kinase, Akt and cell survival. In: Seminars in cell & developmental biology, vol. 15. Cambridge, MA: Academic Press; 2004. p. 177–82.
Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Shahbaz M, Qaisrani TB, Shah ZA, Plygun S, Heydari M. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019;235:116797.
Morissette M, Litim N, Di Paolo T. Natural phytoestrogens: a class of promising neuroprotective agents for Parkinson disease. In: Discovery and development of neuroprotective agents from natural products. Amsterdam: Elsevier; 2018. p. 9–61.
Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharm. 2001;33(1):2–16.
Mehdi SH, Nafees S, Zafaryab M, Khan MA, Alam Rizvi MM. Chrysin: a promising anticancer agent its current trends and future perspectives. Eur J Exp Biol. 2018;8:16.
Sun LR, Zhou W, Zhang HM, Guo QS, Yang W, Li BJ, Sun ZH, Gao SH, Cui RJ. Modulation of multiple signaling pathways of the plant-derived natural products in cancer. Front Oncol. 2019;9:1153.
Siddiqui A, Badruddeen, Akhtar J, Uddin MS S, Khan MI, Khalid M, Ahmad M. A naturally occurring flavone (Chrysin): chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J Biol Active Prod Nat. 2018;8(4):208-227.
Santos BL, Oliveira MN, Coelho PL, Pitanga BP, da Silva AB, Adelita T, Silva VD, de FD Costa M, El-Bachá RS, Tardy M, Chneiweiss H. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem Biol Interact. 2015;242:123–38.
Hong TB, Rahumatullah A, Yogarajah T, Ahmad M, Yin KB. Potential effects of chrysin on MDA-MB-231 cells. Int J Mol Sci. 2010;11(3):1057–69.
Yang F, Jin H, Pi J, Jiang JH, Liu L, Bai HH, Yang PH, Cai JY. Anti-tumor activity evaluation of novel chrysin–organogermanium (IV) complex in MCF-7 cells. Bioorg Med Chem Lett. 2013;23(20):5544–51.
Weng MS, Ho YS, Lin JK. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: involvement of p38 mitogen-activated protein kinase. Biochem Pharmacol. 2005;69(12):1815–27.
Wang J, Wang H, Sun K, Wang X, Pan H, Zhu J, Ji X, Li X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des Dev Ther. 2018;12:721.
Souza LC, Antunes MS, Borges Filho C, Del Fabbro L, de Gomes MG, Goes AT, Donato F, Prigol M, Boeira SP, Jesse CR. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav. 2015;134:22–30.
Gülden M, Appel D, Syska M, Uecker S, Wages F, Seibert H. Chrysin and silibinin sensitize human glioblastoma cells for arsenic trioxide. Food Chem Toxicol. 2017;105:486–97.
Markiewicz-Żukowska R, Borawska MH, Fiedorowicz A, Naliwajko SK, Sawicka D, Car H. Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement Altern Med. 2013;13(1):1–9.
Liao CL, Chen CM, Chang YZ, Liu GY, Hung HC, Hsieh TY, Lin CL. Pine (pinus morrisonicola hayata) needle extracts sensitize GBM8901 human glioblastoma cells to temozolomide by downregulating autophagy and O 6-methylguanine-DNA methyltransferase expression. J Agric Food Chem. 2014;62(43):10458–67.
Borawska MH, Naliwajko SK, Moskwa J, Markiewicz-Żukowska R, Puścion-Jakubik A, Soroczyńska J. Anti-proliferative and anti-migration effects of Polish propolis combined with Hypericum perforatum L. on glioblastoma multiforme cell line U87MG. BMC Complement Altern Med. 2016;16(1):1–9.
Mani R, Natesan V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–96.
Eatemadi A, Daraee H, Aiyelabegan HT, Negahdari B, Rajeian B, Zarghami N. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed Pharmacother. 2016;84:1915–22.
Zheng H, Li S, Pu Y, Lai Y, He B, Gu Z. Nanoparticles generated by PEG-Chrysin conjugates for efficient anticancer drug delivery. Eur J Pharm Biopharm. 2014;87(3):454–60.
Sabzichi M, Mohammadian J, Bazzaz R, Pirouzpanah MB, Shaaker M, Hamishehkar H, Chavoshi H, Salehi R, Samadi N. Chrysin loaded nanostructured lipid carriers (NLCs) triggers apoptosis in MCF-7 cancer cells by inhibiting the Nrf2 pathway. Process Biochem. 2017;60:84–91.
Anari E, Akbarzadeh A, Zarghami N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif Cells Nanomed Biotechnol. 2016;44(6):1410–6.
Mohammadian F, Pilehvar-Soltanahmadi Y, Mofarrah M, Dastani-Habashi M, Zarghami N. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44(8):1972–8.
Kikuta S. The cytotoxic effect of genistein, a soybean isoflavone, against cultured tribolium cells. Insects. 2020;11(4):241.
Kaufman PB, Duke JA, Brielmann H, Boik J, Hoyt JE. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health. J Altern Complement Med. 1997;3(1):7–12.
Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62(7):996–1000.
Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–80.
Singh-Gupta V, Joiner MC, Runyan L, Yunker CK, Sarkar FH, Miller S, Gadgeel SM, Konski AA, Hillman GG. Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J Thorac Oncol. 2011;6(4):688–98.
Sobhy MM, Mahmoud SS, El-Sayed SH, Rizk EM, Raafat A, Negm MS. Impact of treatment with a protein tyrosine kinase inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp Parasitol. 2018;185:115–23.
Puli S, Jain A, Lai JC, Bhushan A. Effect of combination treatment of rapamycin and isoflavones on mTOR pathway in human glioblastoma (U87) cells. Neurochem Res. 2010;35(7):986–93.
Khoshyomn S, Nathan D, Manske GC, Osler TM, Penar PL. Synergistic effect of genistein and BCNU on growth inhibition and cytotoxicity of glioblastoma cells. J Neurooncol. 2002;57(3):193–200.
Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–65.
Schmidt F, Knobbe CB, Frank B, Wolburg H, Weller M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep. 2008;19(4):1061–6.
Wang HK. The therapeutic potential of flavonoids. Expert Opin Investig Drugs. 2000;9(9):2103–19.
Myers DE, Sicheneder A, Clementson D, Dvorak N, Venkatachalam T, Sev AR, Chandan-Langlie M, Uckun FM. Large scale manufacturing of B43 (anti-CD19)-genistein for clinical trials in leukemia and lymphoma. Leuk Lymphoma. 1998;29(3-4):329–38.
Mendes LP, Gaeti MP, de Ávila PH, de Sousa VM, dos Santos RB, de Ávila Marcelino RI, Dos Santos LC, Valadares MC, Lima EM. Multicompartimental nanoparticles for co-encapsulation and multimodal drug delivery to tumor cells and neovasculature. Pharm Res. 2014;31(5):1106–19.
Chuan LI, Zhang J, Yu-Jiao ZU, Shu-Fang NI, Jun CA, Qian WA, Shao-Ping NI, Ze-Yuan DE, Ming-Yong XI, Shu WA. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin J Nat Med. 2015;13(9):641–52.
de Azambuja CR, dos Santos LG, Rodrigues MR, Rodrigues RF, da Silveira EF, Azambuja JH, Flores AF, Horn AP, Dora CL, Muccillo-Baisch AL, Braganhol E. Physico-chemical characterization of asolectin–genistein liposomal system: An approach to analyze its in vitro antioxidant potential and effect in glioma cells viability. Chem Phys Lipids. 2015;193:24–35.
Phan V, Walters J, Brownlow B, Elbayoumi T. Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. J Drug Target. 2013;21(10):1001–11.
de Azambuja Borges CR, Silva NO, Rodrigues MR, Marinho MA, de Oliveira FS, Cassiana M, Horn AP, Parize AL, Flores DC, Clementin RM, de Lima VR. Dimiristoylphosphatidylcholine/genistein molecular interactions: a physico-chemical approach to anti-glioma drug delivery systems. Chem Phys Lipids. 2019;225:104828.
Puli S, Lai JC, Bhushan A. Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J Neurooncol. 2006;79(2):135–42.
Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62(7):996–1000.
Goodman M, Bostick RM, Kucuk O, Jones DP. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic Biol Med. 2011;51(5):1068–84.
Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–42.
Singh-Gupta V, Joiner MC, Runyan L, Yunker CK, Sarkar FH, Miller S, Gadgeel SM, Konski AA, Hillman GG. Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J Thorac Oncol. 2011;6(4):688–98.
Puli S, Jain A, Lai JC, Bhushan A. Effect of combination treatment of rapamycin and isoflavones on mTOR pathway in human glioblastoma (U87) cells. Neurochem Res. 2010;35(7):986–93.
Kim KH, Seol HJ, Kim EH, Rheey J, Jin HJ, Lee Y, Joo KM, Lee J, Nam DH. Wnt/β-catenin signaling is a key downstream mediator of MET signaling in glioblastoma stem cells. Neuro Oncol. 2013;15(2):161–71.
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie SM, Du B, Zhong XY. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J Neurooncol. 2015;121(1):41–52.
Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, Hofman FM, Louie SG, Schönthal AH. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302(2):100–8.
Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Ahmad N, Wood GS. (-)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle. 2009;8(13):2057–63.
Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp Cell Res. 2013;319(5):697–706.
Lee HK, Xiang C, Cazacu S, Finniss S, Kazimirsky G, Lemke N, Lehman NL, Rempel SA, Mikkelsen T, Brodie C. GRP78 is overexpressed in glioblastomas and regulates glioma cell growth and apoptosis. Neuro Oncol. 2008;10(3):236–43.
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–21.
Liang G, Tang A, Lin X, Li L, Zhang S, Huang Z, Tang H, Li QQ. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol. 2010;37(1):111–23.
Li C, Zhou C, Wang S, Feng Y, Lin W, Lin S, Wang Y, Huang H, Liu P, Mu YG, Shen X. Sensitization of glioma cells to tamoxifen-induced apoptosis by Pl3-kinase inhibitor through the GSK-3β/β-catenin signaling pathway. PLoS One. 2011;6(10):e27053.
Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234–40.
Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CY, Flynn PJ. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97(6):1442–6.
Shanafelt TD, Lee YK, Call TG, Nowakowski GS, Dingli D, Zent CS, Kay NE. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk Res. 2006;30(6):707–12.
Manero F, Gautier F, Gallenne T, Cauquil N, Grée D, Cartron PF, Geneste O, Grée R, Vallette FM, Juin P. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66(5):2757–64.
Meng Y, Lyu F, Xu X, Zhang L. Recent advances in chain conformation and bioactivities of triple-helix polysaccharides. Biomacromolecules. 2020;21(5):1653–77.
Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9(2):196–223.
Bae IY, Kim HW, Yoo HJ, Kim ES, Lee S, Park DY, Lee HG. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Res Int. 2013;51(1):195–200.
Li GH, Shen YM, Zhang KQ. Nematicidal activity and chemical component of Poria cocos. J Microbiol. 2005;43(1):17–20.
Cheng JJ, Chang CC, Chao CH, Lu MK. Characterization of fungal sulfated polysaccharides and their synergistic anticancer effects with doxorubicin. Carbohydr Polym. 2012;90(1):134–9.
Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169(9):5270–8.
Atashrazm F, Lowenthal RM, Woods GM, Holloway AF, Dickinson JL. Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Mar Drugs. 2015;13(4):2327–46.
Zhang Y, Li S, Wang X, Zhang L, Cheung PC. Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011;25(2):196–206.
Zhou Z, Han Z, Zeng Y, Zhang M, Cui Y, Xu L, Zhang L. Chinese FDA approved fungal glycan-based drugs: an overview of structures, mechanisms and clinical related studies. Transl Med. 2014;4(141):1.
Yoshino S, Tabata T, Hazama S, Iizuka N, Yamamoto K, Hirayama M, Tangoku A, Oka M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000;20(6C):4707–11.
Wang XE, Wang YH, Zhou Q, Peng M, Zhang J, Chen M, Ma LJ, Xie GM. Immunomodulatory effect of lentinan on aberrant T subsets and cytokines profile in non-small cell lung cancer patients. Pathol Oncol Res. 2020;26(1):499–505.
Xie JH, Jin ML, Morris GA, Zha XQ, Chen HQ, Yi Y, Li JE, Wang ZJ, Gao J, Nie SP, Shang P. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr. 2016;56(sup1):S60–84.
Deng S, Zhang G, Kuai J, Fan P, Wang X, Zhou P, Yang D, Zheng X, Liu X, Wu Q, Huang Y. Lentinan inhibits tumor angiogenesis via interferon γ and in a T cell independent manner. J Exp Clin Cancer Res. 2018;37(1):1–2.
Higashi D, Seki K, Ishibashi Y, Egawa Y, Koga M, Sasaki T, Hirano K, Mikami K, Futami K, Maekawa T, Sudo M. The effect of lentinan combination therapy for unresectable advanced gastric cancer. Anticancer Res. 2012;32(6):2365–8.
Zhou B, Fu Q, Song SS, Zheng HL, Wei YZ. Inhibitory effect of schizophyllan on rat glioma cells. Bangladesh J Pharmacol. 2015;10(4):759–64.
Ina H, Yoneda M, Kanda M, Kodera Y, Kabeya M, Yuasa S. Lentinan, a shiitake mushroom beta-glucan, stimulates tumor-specific adaptive immunity through PD-L1 down-regulation in gastric cancer cells. Med Chem. 2016;6:710–4.
Mawson AR. Retinoids in the treatment of glioma: a new perspective. Cancer Manag Res. 2012;4:233.
Haar CP, Hebbar P, Wallace GC, Das A, Vandergrift WA, Smith JA, Giglio P, Patel SJ, Ray SK, Banik NL. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37(6):1192–200.
Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130(2):479S–82S.
Das A, Banik NL, Ray SK. N-(4-Hydroxyphenyl) retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res. 2008;1227:207–15.
Mellai M, Caldera V, Patrucco A, Annovazzi L, Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res. 2008;28(1A):109–18.
George J, Banik NL, Ray SK. Survivin knockdown and concurrent 4-HPR treatment controlled human glioblastoma in vitro and in vivo. Neuro Oncol. 2010;12(11):1088–101.
Tian W, Deng Z, Hong K. The biological activities of sesterterpenoid-type ophiobolins. Mar Drugs. 2017;15(7):229.
Bury M, Novo-Uzal E, Andolfi A, Cimini S, Wauthoz N, Heffeter P, Lallemand B, Avolio F, Delporte C, Cimmino A, Dubois J. Ophiobolin A, a sesterterpenoid fungal phytotoxin, displays higher in vitro growth-inhibitory effects in mammalian than in plant cells and displays in vivo antitumor activity. Int J Oncol. 2013;43(2):575–85.
Bury M, Girault A, Megalizzi V, Spiegl-Kreinecker S, Mathieu V, Berger W, Evidente A, Kornienko A, Gailly P, Vandier C, Kiss R. Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis. 2013;4(3):e561.
Morrison R, Lodge T, Evidente A, Kiss R, Townley H. Ophiobolin A, a sesterpenoid fungal phytotoxin, displays different mechanisms of cell death in mammalian cells depending upon the cancer cell origin. Int J Oncol. 2017;50(3):773–86.
Brunelli D, Tavecchio M, Falcioni C, Frapolli R, Erba E, Iori R, Rollin P, Barillari J, Manzotti C, Morazzoni P, D’Incalci M. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem Pharmacol. 2010;79(8):1141–8.
Prawan A, Saw CL, Khor TO, Keum YS, Yu S, Hu L, Kong AN. Anti-NF-κB and anti-inflammatory activities of synthetic isothiocyanates: effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179(2–3):202–11.
Subedi L, Venkatesan R, Kim SY. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling. Int J Mol Sci. 2017;18(7):1423.
Guo Z, Wang H, Wei J, Han L, Li Z. Sequential treatment of phenethyl isothiocyanate increases sensitivity of Temozolomide resistant glioblastoma cells by decreasing expression of MGMT via NF-κB pathway. Am J Transl Res. 2019;11(2):696.
Lee CS, Cho HJ, Jeong YJ, Shin JM, Park KK, Park YY, Bae YS, Chung IK, Kim M, Kim CH, Jin F. Isothiocyanates inhibit the invasion and migration of C6 glioma cells by blocking FAK/JNK-mediated MMP-9 expression. Oncol Rep. 2015;34(6):2901–8.
Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J Nanosci Nanotechnol. 2004;4(5):484–8.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Peptide transport and delivery into the central nervous system. 2003; pp. 39-78.
Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–74.
Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90.
Egleton RD, Davis TP. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx. 2005;2(1):44–53.
Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med. 2009;6(34):139–43.
Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. 2014;4:126.
Kim SS, Rait A, Rubab F, Rao AK, Kiritsy MC, Pirollo KF, Wang S, Weiner LM, Chang EH. The clinical potential of targeted nanomedicine: delivering to cancer stem-like cells. Mol Ther. 2014;22(2):278–91.
Panyam J, Labhasetwar V. Sustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticles. Mol Pharm. 2004;1(1):77–84.
Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30(12):2302–18.
Li Y, He H, Jia X, Lu WL, Lou J, Wei Y. A dual-targeting nanocarrier based on poly (amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–908.
Hendricks BK, Cohen-Gadol AA, Miller JC. Novel delivery methods bypassing the blood-brain and blood-tumor barriers. Neurosurg Focus. 2015;38(3):E10.
Lai JC, Ananthakrishnan G, Jandhyam S, Dukhande VV, Bhushan A, Gokhale M, Daniels CK, Leung SW. Treatment of human astrocytoma U87 cells with silicon dioxide nanoparticles lowers their survival and alters their expression of mitochondrial and cell signaling proteins. Int J Nanomedicine. 2010;5:715.
Bobyk L, Edouard M, Deman P, Vautrin M, Pernet-Gallay K, Delaroche J, Adam JF, Estève F, Ravanat JL, Elleaume H. Photoactivation of gold nanoparticles for glioma treatment. Nanomed Nanotechnol Biol Med. 2013;9(7):1089–97.
Aryal M, Park J, Vykhodtseva N, Zhang YZ, McDannold N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: evaluation during tumor progression in a rat glioma model. Phys Med Biol. 2015;60(6):2511.
Kim SS, Harford JB, Pirollo KF, Chang EH. Effective treatment of glioblastoma requires crossing the blood–brain barrier and targeting tumors including cancer stem cells: the promise of nanomedicine. Biochem Biophys Res Commun. 2015;468(3):485–9.
Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13(23-24):1099–106.
Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD, Gelperina SE. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759–67.
Zhang Y, Sun C, Kohler N, Zhang M. Self-assembled coatings on individual monodisperse magnetite nanoparticles for efficient intracellular uptake. Biomed Microdevices. 2004;6(1):33–40.
Eldridge B. Preclinical development of carbon nanotube mediated thermal therapy for treatment of glioblastoma multiforme: Determining the effects of nanomaterial properties, dose, and irradiation parameters on the efficacy and potential negative repercussions of treatment (Doctoral dissertation, Wake Forest University).
Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, Komotar RJ. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev Anticancer Ther. 2013;13(12):1453–61.
Panigrahi M, Das PK, Parikh PM. Brain tumor and Gliadel wafer treatment. Indian J Cancer. 2011;48(1):11.
Miglierini P, Bouchekoua M, Rousseau B, Hieu PD, Malhaire JP, Pradier O. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114(9):1222–5.
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88.
Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345(8956):1008–12.
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quiñones-Hinojosa A. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–8.
Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3(5):721–8.
Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol. 2005;7(1):84–9.
Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, Sailor MJ. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci. 2007;104(3):932–6.
Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor–targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5(12):3162–9.
Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW. Epidermal growth factor receptor–targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–8.
Lee YE, Kopelman R. Polymeric nanoparticles for photodynamic therapy. In: Biomedical nanotechnology. Totowa, NJ: Humana Press; 2011. p. 151–78.
Jain KK. Potential of nanobiotechnology in the management of glioblastoma multiforme. In: Glioblastoma. New York, NY: Springer; 2010. p. 399–419.
Koo YE, Reddy GR, Bhojani M, Schneider R, Philbert MA, Rehemtulla A, Ross BD, Kopelman R. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev. 2006;58(14):1556–77.
Gupta AH, Kathpalia HT. Recent advances in brain targeted drug delivery systems: a review. Int J Pharm Pharm Sci. 2014;6(2):51–7.
Hostanska K, Reichling J, Bommer S, Weber M, Saller R. Hyperforin a constituent of St John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm. 2003;56(1):121–32.
Ferguson PJ, Kurowska EM, Freeman DJ, Chambers AF, Koropatnick J. In vivo inhibition of growth of human tumor lines by flavonoid fractions from cranberry extract. Nutr Cancer. 2006;56(1):86–94.
Lee DY, Lee MK, Kim GS, Noh HJ, Lee MH. Brazilin inhibits growth and induces apoptosis in human glioblastoma cells. Molecules. 2013;18(2):2449–57.
Lee DH, Lee TH, Jung CH, Kim YH. Wogonin induces apoptosis by activating the AMPK and p53 signaling pathways in human glioblastoma cells. Cell Signal. 2012;24(11):2216–25.
Li Y, Zhang P, Qiu F, Chen L, Miao C, Li J, Xiao W, Ma E. Inactivation of PI3K/Akt signaling mediates proliferation inhibition and G2/M phase arrest induced by andrographolide in human glioblastoma cells. Life Sci. 2012;90(25-26):962–7.
Khan M, Yu B, Rasul A, Al Shawi A, Yi F, Yang H, Ma T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid Based Complement Alternat Med. 2012;2012:703034.
Chang LF, Lin PC, Ho LI, Liu PY, Wu WC, Chiang IP, Chang HW, Lin SZ, Harn YC, Harn HJ, Chiou TW. Overexpression of the orphan receptor Nur77 and its translocation induced by PCH4 may inhibit malignant glioma cell growth and induce cell apoptosis. J Surg Oncol. 2011;103(5):442–50.
Chaudhuri D, Ghate NB, Singh SS, Mandal N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn Mag. 2015;11(42):269.
Zhang Y, Xie RF, Xiao QG, Li R, Shen XL, Zhu XG. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J Ethnopharmacol. 2014;158:404–11.
Tezcan G, Tunca B, Bekar A, Budak F, Sahin S, Cecener G, Egeli U, Taskapılıoglu MO, Kocaeli H, Tolunay S, Malyer H. Olea europaea leaf extract improves the treatment response of GBM stem cells by modulating miRNA expression. Am J Cancer Res. 2014;4(5):572.
Elkady AI, Hussein RA, Abu-Zinadah OA. Effects of crude extracts from medicinal herbs Rhazya stricta and Zingiber officinale on growth and proliferation of human brain cancer cell line in vitro. Biomed Res Int. 2014;2014:260210.
U.S. National Library of Medicine. ClinicalTrials.gov [Internet]. U.S. National Institute of Health. 2020 [cited 2020 Dec 2]. Available from: https://clinicaltrials.gov/ct2/home
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Siddiqui, E.M., Khan, A., Mehan, S., Sahu, R. (2021). Green Nanoparticles: A Hope for Targeted Delivery of Natural Therapeuticals for the Management of Glioblastoma Multiforme (GBM). In: Tabrez, S., Imran Khan, M. (eds) Polyphenols-based Nanotherapeutics for Cancer Management. Springer, Singapore. https://doi.org/10.1007/978-981-16-4935-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-4935-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4934-9
Online ISBN: 978-981-16-4935-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)