Abstract
Orofacial region and dental tissues harbour a wide range of stem/progenitor cells including mesenchymal stem cells and tissue-specific progenitors such as muscle satellite cells. The stem cells of the orofacial areas are readily available, highly proliferative and possess multi-differentiation abilities. These cells not only provide therapeutic and tissue engineering cell source for the defects of orofacial area and dental tissues but also provide additional cell source for the diseases of other organs. Understanding their differentiation pathways and mechanisms will be imperative in developing the most appropriate approaches for stem cell-based tissue engineering and therapeutic strategies.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Overview
1.1 Need for Stem/Progenitor Cell-Based Therapy
Stem cells are defined as the cells that have two important abilities: (1) self-renewal and (2) differentiation. They have remarkable potential to develop into many different cell types in the body during early life and growth [1]. In adults, they serve as a cell source for continuous repair system, dividing essentially without limit to replenish other cells as long as the person or animal is still alive, as best exampled with the haematopoietic system, bone marrow (BM) stem cells providing blood cells throughout a person’s life. Other adult organs are less regenerative in comparison; still, minor wears and tears are constantly repaired with stem cells by providing broader matrix turnover and cellular replenishment or by exerting more specialized function, such as differentiating into muscle cells, neurons, or, in the dental context, odontoblasts.
However, in adult mammalians, this reparative ability seems to be limited to minor injuries. In catastrophic events such as myocardial infarction (MI), spinal cord injury (SCI) or autoimmune and degenerative diseases, the endogenous stem cells do not seem to have the capacity to provide sufficient support for the parenchymal tissue regeneration. The lost tissue is often replaced by fibrous scar, compromising the integrity and function of the damaged organs. Therefore, developing approaches that can either activate the endogenous stem cells in vivo or expand them in vitro for transplantation still hold major research interest in the regenerative medicine.
Stem and progenitor cells exert their therapeutic abilities mainly through two mechanisms:
-
1.
Functional cellular replacement of the damaged organs, for example, replacement of the neurons in SCI or replacement of the cardiomyocytes after MI. This aspect is the most desirable property of the stem cell therapeutics but currently is also difficult to achieve with adult stem cell sources. Embryonic stem (ES) cells and the induced pluripotent promise the pluripotency that can be used to differentiate various cell types for transplantation, however with caveats including uncontrolled cell proliferations and teratogenicity limiting the clinical use of these cells.
-
2.
Modulation of the repair processes by providing extracellular matrix remodelling, paracrine trophic factors and immune modulation. More often than not, and in the mesenchymal stem cell (MSC) therapies in particular, the trophic effects seem to play a major role in the improvement of the organ function by modulating vascular supply and maintenance of tissue architectures rather than providing engraftment of the parenchymal cell types. The immunomodulatory effects of the MSCs have been exploited to treat various diseases including cardiac injuries and autoimmune diseases, with cells isolated and investigated from virtually all organs and tissues in the body.
Studies of the orofacial stem cells have been relatively newer events. Majority of the orofacial stem cell studies have been focused on the MSC cell types of the dental pulp, periodontal ligaments, facial BM, submucosal stromal and tongue. In the meantime, studies include generation of the parenchymal cell types of salivary glands, and recent speculation of the possible use of the tongue myogenic progenitors for cardiac repair also hold intriguing perspectives for future exploration of the stem cells in the orofacial region.
Orofacial region provides advantages over other cell sources:
-
1.
Easy accessibility: Cells can be obtained from extracted third molars (pulp and periodontal cell sources), exfoliated deciduous teeth, oral mucosa biopsies and tissues that are extractable without major invasive procedures compared to other sources.
-
2.
Higher potency: For example, MSCs isolated from the dental sources may possess high potencies compared to BM source as discussed below.
-
3.
Complexity: Multi-lineage derivation of the orofacial tissues that contain all three germ layers of embryonic origins (endoderm, ectoderm and mesoderm, as discussed below) that may provide expanded versatility for stem cell therapies.
In this chapter, we discuss the developmental cell sources of the orofacial areas focusing on the discussion of the MSCs derived from orofacial region and their utility in the local and systemic diseases, as well as the potential promise of the orofacial muscle progenitor usage for cardiac regenerations.
1.2 Cell Sources During Orofacial Development
During vertebrate development, including humans, the oral and facial region develops very early in the embryos following formation of the cardiac tube and folding of the neural tube, approximately at the end of week 3 of prenatal life. The earliest orofacial progenitors originate posteriorly to the heart field and contain vascular (aortic arch) and muscular progenitors, which are joined by the neural crest mesenchymal (also known as ectomesenchyme) progenitor migration as the neural tube closes [2]. Expansion of these two populations facilitates the formation of the first and second pharyngeal arches that provide initial myogenic progenitors for orofacial musculatures, including masticatory, facial expressions and tongue [3, 4], and the multi-fated neural crest progenitors for formation of facial cartilage, flat bones, teeth and tooth support tissues such as periodontal ligaments [5]. Recent studies indicate that, albeit to a limited extent, adult tissues may retain some of these stem/progenitor cells at various stages of the development, become dormant and, when activated, are able to achieve minor repairs of the tissues during injury. Indeed, studies have shown the MSCs isolated from the orofacial area are neural crest in origin [6] and may have different differentiation potential compared to the MSCs isolated from other tissues such as the BM and heart which are thought to have been derived from the mesodermal origin [7].
At the later stages of the embryonic development, the mesodermal muscle progenitors (paraxial/occipital) and mesenchymal stromal cells aid the expansion of the muscle cell pools and the connective tissue (including bone) maturations. However, these cell sources provoke less interest from orofacial stem cell research point of view as the clonogenic stem cells isolated from this region seem to be more neural crest in origin [8].
The pharyngeal arches that give rise to orofacial tissues are internally lined by the endodermal epithelium and externally covered by the ectodermal epithelium; both form the oral mucosa lining as well as give rise to salivary glands [9]. Isolation of the myoepithelial progenitors from the salivary glands has shown to generate organoids in vitro and is able to engraft in the irradiated mice to form functional salivary glands [10].
2 Mesenchymal Stem Cell-Derived Oral Tissues
2.1 Markers and Sources of Orofacial MSCs
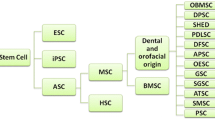
Majority of the orofacial stem cell research involves isolation of the so-called MSC population from various tissues. MSCs are first isolated by Friedenstain from BM and generally described as a group of plastic adherent cells that assume a spindle-shaped morphology and form so-called colony-forming unit fibroblasts (CFU-F), which can differentiate into connective tissue cells (adipocytes, osteocytes and chrondrocytes)in vitro [11]. In the orofacial region, the MSCs are isolated from adult human dental pulp, pulp of the exfoliated deciduous teeth, periodontal ligaments, apical follicles, mandibular BM and oral mucosal connective tissues that can be transplanted to various organs.
Similar to the MSCs derived from other tissues, orofacial MSCs do not display any tissue-specific markers in vivo. Some of the markers of in vitro derivatives of dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLCs) include stromal markers such as CD90, CD44, CD13, CD19 and CD79 and pericyte markers such as CD146. In mouse, the MSCs are marked within a broader stromal population of the stem cell antigen 1 (Sca-1)-positive cohorts. In humans, Stro-1 and CD34 were described as MSC makers that can be used to isolate the in vitroCFU-F forming cells, albeit all these markers are expressed by other cell types and highly unspecific. Recently, platelet-derived growth factor receptor alpha (PDGFRa) has been described as a possible in vivomarker for the enrichment of MSC CFU-F forming ability in vitro and engraftment in vivo [12, 13]. Studies have shown that the PDGFRa expresses broadly in the neural crest cells, [14] and in the dental organs, clonogenic MSCs can be isolated from the dental pulp tissue using this marker [8]. However, no comprehensive enrichment studies have been conducted using PDGFRa . Similar to other MSCs, these cells generally lack haematopoietic cell markers such as CD45 and CD31 [1, 15].
Orofacial MSCs cells originate from the embryonic neural crest, generally described as possessing higher differentiation potencies than the MSCs isolated from other sites [16]. For example, MSCs isolated from the mandibular bone can engraft and regenerate tibial defect but the reverse is not possible. Dental pulp MSCs express embryonic markers that are not expressed in the MSCs isolated from other tissues as detailed below. This is partially due to a cordial form of Hox gene expression in these cells compared to the mesoderm-derived MSCs [17].
The presence of adult MSC populations within orofacial tissue, and their ability to adopt tissue-specific phenotypes, given the appropriate differentiation conditions, has led many investigators to suggest that the primary role of MSCs is to serve as cell replacement during the natural course of tissue turnover and homeostasis [18]. In addition, MSCs may serve important therapeutic roles because they appear to escape immune recognition and exert anti-inflammatory and immunomodulatory effects via the suppression of T, B, natural killer and antigen-presenting cells, both in vitro and in vivo. The immunomodulatory properties of these cells, and the ability to isolate and expand them in vitrowithout loss of their phenotypic or multi-lineage potential, have generated great interest in using MSCs as a therapeutic modality for immune-mediated diseases and tissue repair [19–21].
2.2 Mesenchymal Stem Cells of Dental Pulp Stem Cells (DPSCs)
Dental pulp tissue is derived from the embryonic dental papilla, which originates from neural crest mesenchymal cells during the morphogenesis of the first pharyngeal arches. DPSCs are presumably inherent descendants of this lineage that occupy a perivascular niche of the dental pulp and possess MSC-like properties [15]. Dental pulp tissue is isolated from coronal pulp of the routinely extracted human teeth or exfoliated deciduous teeth, therefore a non-invasive process. DPSCs have shown to generate bone when they are implanted in mice [22]; deciduous teeth MSCs were found to be able to induce bone formation, generate dentin and survive in mouse brain along with expression of neural markers that appear to be different from previously identified stem cells such as from BM [23].
In culture, DPSCs have been shown to express ES cell markers not currently identified in other adult MSCs; these markers include Oct-4, Nanog, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 [24], and their presence may signify higher potency and enhanced stem cell activity for DSPCs compared to other adult tissue-derived stem cells. They can differentiate into dental structures, as well as smooth and skeletal muscles, neurons, cartilage and bone under defined in vitroculture conditions [15, 25], engraft to muscular dystrophic skeletal muscle and improve post-myocardial infarction cardiac function in animal models although without evidence of engraftment [26, 27].
Notably, recent reports further speculated that the DPSCs might have originated from a neuroectodermal lineage. Indeed, the dental pulp stem cells express neural markers, and they can differentiate into functionally active neurons [28–30] and, when stimulated, express neurogenic substance such as glutamate. Transplantation of human DPSCs into rat SCI model has shown marked recovery of hind limb locomotor functions via improved preservation of neuronal filaments and myelin sheaths by inhibition of apoptosis, promotion of regeneration of transected axons through paracrine mechanisms and replacement of lost cells by differentiating into mature oligodendrocytes [31].
2.3 MSCs of the Periodontal Ligament, Apical Papilla and Follicles
Periodontal tissue contains both hard and soft tissues. Periodontal tissue ligaments are and gingival tissues are derived neural crest mesenchyme reside in the dental follicles during tooth development. Similar to the dental pulp and BM MSCs, the periodontal ligament stem cells (PDLSCs) are also thought to be perivascular. Clonally selected Stro-1 and CD146 as markers have been isolated from extracted human third molars and shown to differentiate into collagen-producing fibrogenic and calcified cementogenic cells in vivo to repair attachment lost in the periodontium in immunosuppressed rats, showing the capacity of these cells to form collagen fibres, similar to Sharpey’s fibres, connected to the cementum-like tissue suggesting the potential to regenerate PDL attachment [32].
Other studies, however, failed to reproduce the osteogenic potential of the PDLSCs. These variations in findings may support the notion that PDLSCs are heterogeneous MSC population that may contain subpopulations that have propensities for either collagen-producing fibrogenic subclones or cement/osteogenic cell types. PDLSC do not form dentin-pulp complexes and instead possess osteoblastic and cementoblastic lineages in order to regenerate periodontal tissue and maintain PDL integrity [33]. Currently, PDLSCs are not currently reported for use in non-dental diseases; it is not clear whether this is due to a higher susceptibility of PDLSCs to periodontal disease states and age related changes.
CFU-F forming cells isolated from the dental apical follicle (DFS) and stem cells of apical papilla (SCAP) of the developing tooth display similar MSC characteristics in vitro, however with perhaps superior differentiation capacity of early/progenitor cells compared to other MSCs from PDL and BM. These cells rapidly attach to the culture dishes, proliferate at a higher rate than other MSCs and express putative stem cell markers such as Notch-1 and nestin, as well as ES cell markers Oct-3/Oct-4, Sox-2 and Nanog reported in SCAP [21, 33]. DFS and SCAP are shown to differentiate into dentine-like structures in in vitro, functional hepatocyte-like cells and neuronal tissue structures (Table 8.1).
3 Muscle Stem Cells
3.1 Potential of Orofacial Muscle Stem Cells in Cardiac Repair
Another intriguing potential source of stem cells may come from the progenitors of the orofacial muscle. The muscles of orofacial area including tongue, facial expression and mastication are classified as skeletal myocytes. These muscles are vital for mammalian survival dictated by their roles in the infant suckling and adult nutritional intake. As well, recent studies have shown that these muscles possess developmental kinship to the cardiac muscle cells that are marked by similar gene expressions such as cardiac-specific transcription factors Isl1and Nkx2-5. The similarities between these two groups also extend to the fatigue resistance of orofacial muscles [36], presumably due to similar glycolytic metabolic properties of the heart and presence of connexin molecules that are similar to cardiac configurations [37]. Unlike the cardiac muscles, the orofacial muscles have a remarkable ability to repair after injury, providing continuous myofibril remodelling throughout life, suggesting an active and proliferative myogenic properties of the satellite cells in these muscles. These attributes suggest that the orofacial muscle progenitors may emerge as an interesting and attractive source for regeneration of myocardium which only heals with non-contractile fibrosis after insults of MI. Thus far, limb muscle progenitors which have been isolated to transplant into myocardium failed to couple with the endogenous cardiomyocytes resulting in arrhythmia [38]. This may be presumably due to the developmental origin and characteristics of the skeletal muscles of the trunk and limbs being distinct from those of the cardiac and facial muscle. Other cell types such as MSCs from the BM and endogenous cardiac source have produced less than optimal results in cardiac stem cell treatments [7, 39], with former resulting in short-term benefits due to trophic effect rather than new cardiomyocyte generation [40] and the latter having limited therapeutic value as it is confined to neonates [41].
3.2 Developmental Similarities of Cardiac and Orofacial Myogenic Progenitors
Recent lineage tracing and clonal analysis have shown that the orofacial and the heart myocytes originate from the multipotent pharyngeal mesoderm cells which ingress through the streak at the same stage [42, 43] and share a distinct lineage kinship in development. Two myogenic linkages have been identified [4]: (1) First, pharyngeal arch lineage gives rise to masticatory muscle and also contributes to myocardial cells in the right ventricle. (2) Second, pharyngeal arch lineage gives rise to muscles of facial expression and contributes myocardial cells to the arterial pole of the heart.
A key gene in early pharyngeal mesoderm genetic programme encodes the LIM homeodomain transcription factor Islet1 (Isl1) which has been shown to identify the multipotent cardiac progenitor cells in the early embryo and the differentiating ES cells that can give rise to myocardial, endothelial and smooth muscle descendants. This gene is also expressed in pharyngeal myogenic progenitor cells and downregulated on differentiation to either cardiac or skeletal muscle fates [41]. Tzahor et al. have shown that pharyngeal myogenic muscle progenitors apparently have exactly the same subset of craniofacial muscles originating from Isl1-expressing progenitor cells [42]. Others have found that treatment of brachiometric satellite cells activates cardiac gene expression including Isl1 and Tbx20. This finding suggests that satellite cells retain the environmental signals of their embryogenic progenitors, highlighting the potential use of these myoblasts for cardiac repair. Interestingly, while no equivalent population to satellite cells is found in cardiac muscle, a small number of residual ISl1-positive cells have been identified and proposed to be resident progenitor cells that may contribute to cardiac growth and repair in the foetal and early postnatal heart [44].
Additionally in our laboratory, initial findings of genetic lineage tracking and protein expression experiments using Nkx2-5, a cardinal cardiomyocyte marker, have shown remarkable similarity of the tongue and masticatory muscle throughout the embryonic development and in adults; as well, the adult tongue has a significant pool of satellite that is marked by the cardiac transcription gene (data not shown), supporting the notion that the activation and cardiogenic differentiation are possible using these cells.
3.3 An Interesting Example of Using Tongue Stem Cell for Cardiac Repair
Shibuya et al., in 2010, characterized for the first time cardiomyocyte-like properties of cultured tongue muscle-derived stem cells. They showed that Sca-1-positive cells isolated from tongue muscles appear to differentiate into cardiomyocytes that electrically cooperate with adjacent cardiomyocytes. They presented the cardiomyocyte phenotype with beating and Nkx2-5 expression. Interestingly, these cells preserve their expression of connexin43 and appear to form gap junctions, as indicated by the transfer of dye and synchronization of calcium transients among adjacent cells. Collectively, these findings strongly suggest tongue progenitors may be ideal for cell therapy in heart disease [37]. Although theirin vitro studies prove promising and their in vivo findings are limited, this may also be due to the markers that are used to isolate the progenitors which may not be targeted to the myogenic progenitors.
Myogenesis including cardiogenesis is complexly orchestrated. Understanding the developmental governance and the regulatory factors that determine the cell fate of the orofacial myocytes may shed light into both the function of the orofacial musculature and the cardiac repair mechanisms.
4 Closing Remark
The interest in organ regeneration using stem cells has increased in the last decade. In this context, orofacial stem cells are promising candidates, as they are readily available and highly proliferative and possess multi-differentiation abilities. These cells not only provide therapeutic and tissue engineering cell source for orofacial area and dental regeneration but also provide additional source for systemic cells.
References
Xaymardan M, et al. Bone marrow stem cell: properties and pluripotency. In: Atala A, Lanza R, editors. Principles of regenerative medicine. San Diego: Elservier; 2008. p. 268–300.
Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220(4594):268–73.
Diogo R, et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature. 2015;520(7548):466–73.
Lescroart F, et al. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137(19):3269–79.
Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130(20):4769–84.
Kaltschmidt B, Kaltschmidt C, Widera D. Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev. 2012;8(3):658–71.
Chong JJ, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9(6):527–40.
Komada Y et al. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. Plos One. 2012;7:e46436.
Rothova M, et al. Lineage tracing of the endoderm during oral development. Dev Dyn. 2012;241(7):1183–91.
Ogawa M, et al. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498.
Friedenstein AJ, et al. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978;6(5):440–4.
Farahani RM, Xaymardan M. Platelet-derived growth factor receptor alpha as a marker of mesenchymal stem cells in development and stem cell biology. Stem Cells Int. 2015;2015:362753.
Houlihan DD, et al. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat Protoc. 2012;7:2103–11.
Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130(3):507–18.
Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704.
Jensen J, et al. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. SICOT J. 2016;2:16.
Creuzet S, et al. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129(18):4301–13.
Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7–8):1198–211.
Ding G, et al. Effect of cryopreservation on biological and immunological properties of stem cells from apical papilla. J Cell Physiol. 2010;223(2):415–22.
Djouad F, et al. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5(7):392–9.
Li Z, et al. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20(1):25–34.
Yang KL, et al. A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy. 2009;11(5):606–17.
Miura M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12.
Kerkis I, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184(3–4):105–16.
Laino G, et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006;17(3):511–5.
Kerkis I, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med. 2008;6:35.
Gandia C, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26(3):638–45.
Nor JE. Tooth regeneration in operative dentistry. Oper Dent. 2006;31(6):633–42.
Thesleff I, Aberg T. Molecular regulation of tooth development. Bone. 1999;25(1):123–5.
Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5(7):499–508.
Sakai K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122(1):80–90.
Zeng W, Wang C, Yang X. Identification of medicinal gualou (fruit-rind of Trichosanthes) and its mixed fruits in Sichuan. Zhongguo Zhong Yao Za Zhi. 1992;17(1):9–12. 62.
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806.
Gomes JA, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51(3):1408–14.
Iohara K, et al. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 2008;26(9):2408–18.
van Eijden TMGJ, Turkawski SJJ. Morphology and physiology of masticatory muscle motor units. Crit Rev Oral Biol Med. 2001;12(1):76–91.
Shibuya M, et al. Tongue muscle-derived stem cells express connexin 43 and improve cardiac remodeling and survival after myocardial infarction in mice. Circ J. 2010;74(6):1219–26.
Abraham MR, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97(2):159–67.
Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S21–6.
Fazel S, et al. Cardioprotective c-kit+cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116(7):1865–77.
Zhou B, et al. Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun. 2008;375(3):450–3.
Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovasc Res. 2011;91(2):196–202.
Tirosh-Finkel L, et al. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133(10):1943–53.
Kelly R. Core issues in craniofacial myogenesis. Exp Cell Res. 2010;316(18):3034–41.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2017 The Author(s)
About this paper
Cite this paper
Xaymardan, M. (2017). Orofacial Stem Cells for Cell-Based Therapies of Local and Systemic Diseases. In: Sasaki, K., Suzuki, O., Takahashi, N. (eds) Interface Oral Health Science 2016. Springer, Singapore. https://doi.org/10.1007/978-981-10-1560-1_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-1560-1_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1559-5
Online ISBN: 978-981-10-1560-1
eBook Packages: MedicineMedicine (R0)