Abstract
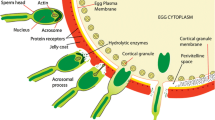
Very little knowledge has been available on structure and function of heavily glycosylated proteins, whose glycan parts amount to more than 90 % of the whole molecules, mainly because of technical difficulties in detection and determination of structure. In 2004 flagellasialin was discovered as a major cell surface component in the lipid rafts of sea urchin sperm. The extremely high content of glycan chains completely prevented detecting this glycoprotein by conventional protein staining methods, such as Coomassie Brilliant Blue and silver staining. To make this glycoprotein visible, monoclonal antibodies 4F7 and 3G9 were developed as specific probes for detecting glycan chains of flagellasialin. The frequent occurrence of GPI-anchored proteins in lipid rafts is well recognized. Flagellasialin was actually demonstrated to contain a GPI-anchor, based on three lines of experiments. In addition to the computational predictions from the cDNA sequence and the phosphatidylinositol-specific phospholipase C treatment of the sperm membrane fraction, nitrous acid deamination of a flagellasialin-blotted membrane was newly introduced. The immunostaining of flagellasialin on the membrane disappeared after the nitrous acid treatment of the blotted membrane. This method was easy and effective for identifying flagellasialin as a GPI-anchored protein.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kambara Y, Shiba K, Yoshida M, Sato C, Kitajima K, Shingyoji C (2011) Mechanism regulating Ca2+-dependent mechanosensory behaviour in sea urchin spermatozoa. Cell Struct Funct 36:69–82
Kasekarn W, Kanazawa T, Hori K, Tsuchiyama T, Xue L, Garénaux E, Kongmanas K, Tanphaichitr N, Yasue H, Sato C, Kitajima K (2012) Pig sperm membrane microdomains contain a highly glycosylated 15–25-kDa wheat germ agglutinin-binding protein. Biochem Biophys Res Commun 426:356–362
Kitajima K, Inoue S, Inoue Y (1989) Isolation and characterization of a novel type of sialoglycoproteins (hyosophorin) from the eggs of medaka, Oryzias latipes: nonapeptide with a large N-linked glycan chain as a tandem repeat unit. Dev Biol 132:544–553
MacRae JI, Ferguson MAJ (2005) A robust and selective method for the quantification of glycosylphosphatidylinositols in biological samples. Glycobiology 15:131–138
Maehashi E, Sato C, Ohta K, Harada Y, Matsuda T, Hirohashi N, Lennarz WJ, Kitajima K (2003) Identification of the sea urchin 350-kDa sperm-binding protein as a new sialic acid-binding lectin that belongs to the heat shock protein 110 family: implication of its binding to gangliosides in sperm lipid rafts in fertilization. J Biol Chem 278:42050–42057
Miyata S, Sato C, Kitamura S, Toriyama M, Kitajima K (2004) A major flagellum sialoglycoprotein in sea urchin sperm contains a novel polysialic acid, an α2,9-linked poly-N-acetylneuraminic acid chain, capped by an 8-O-sulfated sialic acid residue. Glycobiology 14:827–840
Miyata S, Sato C, Kumita H, Toriyama M, Vacquier VD, Kitajima K (2006) Flagellasialin: a novel sulfated α2,9-linked polysialic acid glycoprotein of sea urchin sperm flagella. Glycobiology 16:1229–1241
Miyata S, Yamakawa N, Toriyama M, Sato C, Kitajima K (2011) Co-expression of two distinct polysialic acids, α2,8- and α2,9-linked polymers of N-acetylneuraminic acid, in distinct glycoproteins and glycolipids in sea urchin sperm. Glycobiology 21:1596–1605
Ohta K, Sato C, Mastuda T, Toriyama M, Lennarz WJ, Kitajima K (1999) Isolation and characterization of low density detergent-insoluble membrane (LD-DIM) fraction from sea urchin sperm. Biochem Biophys Res Commun 258:616–623
Ohta K, Sato C, Matsuda T, Toriyama M, Vacquier VD, Lennarz WJ, Kitajima K (2000) Co-localization of receptor and transducer proteins in the glycosphingolipid-enriched, low density, detergent-insoluble membrane fraction of sea urchin sperm. Glycoconj J 17:205–214
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Kanazawa, T., Suzuki, E., Miyata, S., Sato, C., Kitajima, K. (2015). Flagellasialin: Highly Glycosylated GPI-Anchored Protein Involved in Intracellular Ca2+ Regulation in Sea Urchin Sperm. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_167
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_167
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences