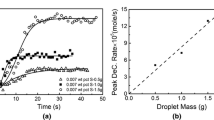
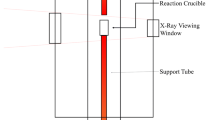
Abstract
Data on the kinetics and thermodynamics of dephosphorization are analyzed in terms of the dynamic partition ratio, determined by a combination of slag thermodynamic properties and the balance of oxygen supply and consumption. A recently developed phosphate capacity correlation is combined with data on kinetics to quantify the most significant parameters in controlling the interfacial oxygen potential. The role of slag composition, entrained gas fraction and temperature are discussed in addition to metal carbon and sulfur content. It is demonstrated that the mass transport of oxygen in the slag is enhanced by factors increasing conductivity and inhibited by entrained gas. The mass transfer of phosphorus in the metal is described considering surface renewal induced by CO bubbles .
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mori K, Fukami Y, Kawai Y (1988) Trans ISIJ 28:315–318
Wei P, Sano M, Hirasawa M, Mori K (1993) ISIJ Int 33:479–487
Monaghan BJ, Pomfret RJ, Coley KS (1998) Metall Trans B 29B:111–118
Shibata E, Sun H, Mori K (1999) Metall Trans B 30B:279–286
Kitamura S, Kitamura T, Shibata K, Mizukami Y, Mukawa S, Nakagawa J (1991) ISIJ Int 31(11):1322–1328
Diao J, Liu X, Zhang T, Xie B (2015) Int J Miner Metall Mater 22(3):249–253
Hewage AK, Rout BK, Brooks G, Naser J (2016) Ironmak Steelmak 43(5):358–370
Manning CP, Fruehan RJ (2013) Metall Trans B 44B:37–44
Miyanoto K, Naito K, Kitagawa I, Matsuo M (2009) Tetsu-to Hagane 95(3):199–206
Wei P, Ohya M, Hirasawa M, Sano M, Mori K (1993) ISIJ Int 33(8):847–854
Gu K, Dogan N, Coley KS (2017) Metall Trans B 48B:2595–2596
Barati M, Coley K (2006) Metall Trans B 37B:51–60
Wiencke J, Lavelaine H, Panteix PJ, Petitjean C, Rapin C (2018) J Appl Electrochem 48(1):115–126
Liu Y, Liu J, Zhang G, Zhang J, Chou K (2018) High Temp. Mater Proc 37(2):121–125
Karimi-Sibaki E, Kharicha A, Wu M, Ludwig A, Bohacek J (2018) Appl Math Comput. https://doi.org/10.1016/j.amc.2018.01.008
Drain PB, Monaghan BJ, Zhang G, Longbottom RJ, Chapman MW, Chew SJ (2017) Ironmak Steelmak 44(10):721–731
Tranell G, Ostrovski O, Jahanshahi S (2002) Metall Trans B 33B:61–67
Liu J, Zhang G, Wu Y, Chou K (2016) Metall Trans B 47B:798–803
Gu K, Dogan N, Coley KS (2017) Metall Trans B 48B:2984–2991
Higbie R (1935) Trans Am Inst Chem Eng 31:365–389
Gu K, Dogan N, Coley KS (2018) Metall Mater Trans B. 49B:1119–1135
Gu K, Dogan N, Coley KS (2018) Steel research international. 89:17450
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Gu, K., Drain, P.B., Monaghan, B.J., Coley, K.S. (2018). Kinetics of Dephosphorization of Iron Carbon Alloys: The Importance of Competing Reactions, Slag Properties and CO Bubbles. In: Davis, B., et al. Extraction 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95022-8_53
Download citation
DOI: https://doi.org/10.1007/978-3-319-95022-8_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95021-1
Online ISBN: 978-3-319-95022-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)