Abstract
A hallmark of disease is that most pathogens are able to infect more than one host species. However, for most pathogens, we still have a limited understanding of how this affects epidemiology, persistence and virulence of infections—including several zoonotic pathogens that reside in wild animal reservoirs and spillover into humans. In this chapter, we review the current knowledge of mallard (Anas platyrhynchos) as host for pathogens. This species is widely distributed, often occupying habitats close to humans and livestock, and is an important game bird species and the ancestor to domestic ducks—thereby being an excellent model species to highlight aspects of the wildlife, domestic animal interface and the relevance for human health. We discuss mallard as host for a range of pathogens but focus more in depth of it as a reservoir host for influenza A virus (IAV). Over the last decades, IAV research has surged, prompted in part to the genesis and spread of highly pathogenic virus variants that have been devastating to domestic poultry and caused a number of human spillover infections. The aim of this chapter is to synthesise and review the intricate interactions of virus, host and environmental factors governing IAV epidemiology and evolution.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Pathogenic microorganisms are a reality for all organisms, with profound impacts on the ecology and evolution of species, populations and individuals (Schmid Hempel 2011). The outcome of infection is variable, ranging from asymptomatic conditions to severe disease and death, depending on factors relating to the host (such as age, immune functions, condition and genetics), the pathogen (such as factors relating to pathogenicity, virulence or transmission) and the environment (e.g. climate, food availability, competition and predation) and their interactions—sometimes described as the pathogen-host-environment matrix (Schmid Hempel 2011). In recent years, a growing understanding has emerged where disease dynamics needs to incorporate the realism of multihost-multipathogen systems—the majority of pathogens can infect more than one host, and most hosts can be infected with several different pathogens (Bordes and Morand 2011).
For humans, it has been estimated that the majority of infectious diseases are zoonotic, e.g. shared with at least one other animal host (Woolhouse and Gowtage-Sequeria 2005). However, we know very little about the dynamics of diseases outside the clinic, even for well-known human diseases such as campylobacteriosis, influenza or rabies, and even less for pathogens that only occasionally spillover into humans. As a response, calls for joint forces, such as the EcoHealth Alliance and One Health initiatives, have been launched, with the aim of bridging the gaps between professionals in human and veterinary medicine and ecology (Roger et al. 2016).
In this chapter, we will focus on the mallard (Anas platyrhynchos), the world’s most numerous wild duck species and the ancestor to domestic ducks, and its role for maintaining pathogens of relevance for humans and domestic animals. Of particular interest is the influenza A virus (IAV), for which the mallard and related waterfowl species are the key reservoir in nature. Because of its great abundance, its expansive distribution and its preference for human-influenced environments, it is a potential bridge species between wild animals, domestic animals and humans, specifically for pathogens to reach domestic ducks and gallinaceous poultry. Moreover, it is an important game species across its distribution, with large bag limits. Most importantly, however, it is one of very few wildlife species from which there is sufficient data to discuss how host ecology affects the disease dynamics in humans and domestic animals. As such, mallard is an important model species for multihost and zoonotic diseases.
2 Waterfowl and Mallard Biology
Waterfowl (order Anseriformes) include ducks, geese and swans and are well known due to their historical importance for hunting, domestication and aviculture. They have a nearly cosmopolitan distribution, except for Antarctica, occupying virtually every habitat associated with water. The approximately 150 species alive today include forms ranging from those occupying highly specialised niches to generalist species able to successfully exploit any wetland habitat, artificial or native. To occupy this wide range of environments, waterfowl are diverse in their anatomy, behaviour and physiology.
Among waterfowl, the mallard is perhaps the archetypical and most recognisable anatid species. Mallards are native to the Holarctic and, however, are thriving globally due to successful introductions to New Zealand, Australia, Peru, Brazil, Uruguay, Argentina, Chile, the Falkland Islands, South Africa and Hawaii (Cramp and Simmons 1977; Drilling et al. 2002). The success of this species is owed to its adaptability; it thrives not only in the wild but is also extremely tolerant of human presence or disturbance and utilises wetlands of all sizes in and around human settlements.
The species is mostly migratory in its native range, but some populations are sedentary with low levels of dispersal. Within migratory populations, there is large flexibility in migratory behaviour, and it is often referred to as being a partial migrant, where some individuals (or populations) are strictly migratory, some strictly residents and others switching between these states depending on specific conditions, such as food availability or cold spells (Cramp et al. 1985; Drilling et al. 2002). It is believed, but rarely tested, that females exhibit a higher degree of philopatry to the natal site than males. As pair formation occurs during late winter at shared wintering sites, this means that a mixing of populations will occur over time. Indeed, this is observed at the genetic level where hardly any population subdivision is evident in either mitochondrial or nuclear genes across the natural breeding distribution of the species (Cramp and Simmons 1977; Drilling et al. 2002; Kraus et al. 2011).
The global mallard population is large, approximately 19 million individuals, of which 7.5 million breed in Europe (Wetlands International 2012). Mortality rates are high, especially during the first year of life, and the turnover rate of mallards in Northern Europe has been estimated to be roughly 1/3, meaning a substantial recruitment rate of young individuals into the population each year (Bentz 1985; Munster and Fouchier 2009). Additionally, large numbers are reared and released for hunting purposes, locally contributing to high densities. It is estimated that 270,000 individuals are released in North America (USFWS 2003, 2011) and greater than 2,850,000 released into Europe annually (Delany and Scott 2006; Birdlife-International 2004; Champagnon 2011). In Eurasia and North America, mallard breeding is mainly restricted to spring and summer resulting in a marked increase in immunologically naïve juveniles in autumn (see Sect. 9.3.2.2).
Nearly all domestic ducks are derived from the mallard, with the exception of Muscovy ducks (Cairina moschata), and domestication dates back to at least the twelfth century (Drilling et al. 2002). Approximately 600 million ducks are farmed in China: 60% of the world population of domestic ducks. As a result, there are more domesticated ducks in China than there are wild mallards across the globe. These large numbers, specifically the large input of young birds, are imperative in the maintenance of infection diseases. Thus, it is a combination of large distribution range, large population size and turnover rate in wild mallards and enormous population size of farmed ducks, most of which are free-range, that make mallard one of the largest aquatic reservoir hosts for diseases. Association with other wild waterfowl, association with farming and domestic poultry and association with humans in urbanised areas make this species extremely important for zoonotic transmission and spillover events.
3 Mallards as Hosts for Pathogenic Microorganisms
Our understanding of avian diseases, particularly in wild birds, is mainly the result of intensive study of poultry diseases, where wildlife can contribute as a source of infection, such as avian influenza (see more below), Newcastle disease (e.g. Kim et al. 2012) or salmonellosis and campylobacteriosis (e.g. Hald et al. 2016; Berglund 2014). Other examples include diseases that cause large mortality events in the wild, such as avian cholera (Samuel et al. 2007) or Wellfleet Bay virus (Allison et al. 2014). It is only more recently, with the onset of molecular methods, that we have started to assess the presence of infectious agents in asymptomatic hosts. For example, recently the tufted duck (Aythya fuligula) microbiome (Strong et al. 2013) and domestic duck viral metagenome (Fawaz et al. 2016) were described. However, the agents we know of are just the tip of the iceberg; for instance, it is predicted that there are 32,000 virus species to be discovered in mammals (Anthony et al. 2013), and it is reasonable to assume that diversity in avian species is in line with that of mammalian hosts. Furthermore, given that we have identified an infectious agent in avian wild birds, we may still know very little about the ecology, epidemiology or even basic biology of these agents.
Wild animal hosts have variable importance to the epidemiology of infectious agents, ranging from optimal or major hosts to minor hosts and to accidental hosts following spillover infections. This is reflected by the adaptation of particular pathogens to their hosts, the efficiency of the parasite to exploit the host effectively for replication and, crucially, the transmission to new hosts. In order to ascertain the specific role of each host species in pathogen epidemiology, it is imperative to combine large screening efforts, molecular-based phylogeny approaches and infection experiments, which unfortunately is rarely met. Compounded with this, we have limited understanding of the role of mallards as hosts for pathogens: are these birds the central reservoir, important but not central to the epidemiology or merely permissive to spillover infection? Moreover, variation in pathogen phenotypic characteristics is usually unknown, such as variation in virulence, pathogenicity, survival in the environment and duration of infection. These properties will depend on both host and pathogen and likely are variable among genetic variants and/or strains of the pathogen. Regardless, our current catalogue of disease-causing agents is probably an underestimation, and with the advent of deep sequencing and more sensitive screening tools, we will likely uncover numerous new disease-causing agents such as picornaviruses (Woo et al. 2010) or disentangle complex epidemiology of known pathogens in new hosts such as coronaviruses (Jackwood et al. 2012).
3.1 Mallards as Hosts for Spillover Infections
An important feature of spillover infections is that the disease typically is not further maintained in the population, because it is not likely transmitted by the spillover host to other hosts, and hence, the disease does not become established within the new population. There are a number of viruses that are shared between wild waterfowl and gallinaceous poultry, of which several are believed to spillover from wild birds into poultry and are of animal health concern, including Newcastle disease virus and infectious bronchitis virus (Table 9.1). It is important to note, however, that transmission can occur also in the other direction, from poultry to wild birds, sometimes associated with wildlife mortality events, as, for instance, noted with duck plague (Converse and Kidd 2001) and avian cholera (Botzler 1991; Gordus 1993) in North America.
Two major sources exist for spillover infections to mallards: spillover from poultry or spillover from other wild birds. Spillover infections from other non-avian hosts are also possible but occur much less frequently or may be underappreciated. First, due to high genetic similarity and sharing habitat with domesticated conspecifics or utilising habitat surrounding intensive poultry farms, there is a high risk for spillover infections from both gallinaceous poultry and domesticated ducks (e.g. Christensen et al. 1998; Wang et al. 2008; Shin et al. 2000, 2002). Indeed, pathogens more frequently found in poultry are identified in wild mallards but very infrequently (Table 9.1). These pathogens are usually only detected in wild mallards utilising habitat surrounding poultry farms as they are used as sentinels. For example, avian pneumovirus has been detected in mallard sentinels and wild mallards in the vicinity of poultry operations (Shin et al. 2000, 2002). Given the large population sizes of birds reared for meat production, one can hypothesise that the occurrence of spillover of poultry-associated pathogens into mallard and other wild bird populations is underestimated. Although modern poultry production units enforce barrier protection, no system can truly be regarded closed—and in many parts of the world, poultry are reared in open units or are let free to roam the environments exposing a large wildlife/domestic animal interface. The extent of this can be exemplified with repeated isolation of bacteria in wild birds with antibiotic resistance profiles suggesting origin in anthropogenic environments (e.g. Stedt et al. 2014; Hasan et al. 2014; Hernandez et al. 2013; Bonnedahl et al. 2014).
An example of spillover from other wild bird species into mallards is West Nile virus (WNV). With the rapid spread of WNV across North America in 2005 and onwards, resulting in wild bird mortality, there was intensive surveillance in numerous bird species (George et al. 2015; LaDeau et al. 2007). Passerine birds were identified as main avian host with spillover into other bird families. These species acquired infection from mosquito vectors but with large interspecies variation in reservoir competence, i.e. in how well the virus could replicate in the birds to achieve sufficient viremia to allow transmission to the vector, and severity of infection. Mallards utilise wetland habitats, so it is not surprising that WNV was detected in this species too (Grard et al. 2007; Lindh et al. 2008; Lobo et al. 2009), but it is not considered a reservoir host. Mallards are also accidentally infected with fungal pathogens due to consuming food laced with fungal spores, such as Aspergillus fumigatus, most notably at times with food shortenings and inclement weather (Adrian et al. 1978; USGS 1999).
The concept of spillover infection, minor host and major host is useful for discussions on the various roles different species can have in the epidemiology of a disease. However, it should be noted that the distinctions are not always clear-cut; rather host type is a continuum starting with occasional individual infections in a new host, continuing to stuttered transmission chains and multihost disease dynamics with increasing specialisation in the different hosts and ending with host-specific transmission and disease (Wolfe et al. 2007; Morse 1995, 2004; Church 2004; Fenton and Pedersen 2005). Importantly, this transgression depends on both the frequency of interspecies transmission and the intraspecies transmission in the novel host given successful interspecies transmission (Fenton and Pedersen 2005). Mallards may act as minor host for many waterfowl diseases due to their association with other waterfowl. Even if repeatedly infected, mallards might not have a central role in the epidemiology of a particular disease. For example, numerous mallards die in outbreaks of the bacterially associated disease avian cholera, also called fowl cholera (Blanchong et al. 2006; Botzler 1991, 2002), but currently these mortality events are occurring in high arctic breeding areas of eiders (Somateria mollissima) (Descamps et al. 2012), Ross’s geese (Chen rossii) and snow geese (Chen caerulescens) (Samuel et al. 2005a, b), and it is geese that are being implicated as long-term carriers of the bacterium (Samuel et al. 2005a, b).
3.2 Mallards as the Main Reservoir: Influenza A Viruses
3.2.1 Influenza A Viruses (IAVs) as a Multihost Pathogen
Influenza A viruses (IAVs) are probably best known for their ability to cause seasonal epidemics and pandemics in humans, such as the pandemic of 1918 Spanish influenza, or the circulating seasonal influenza. They are, however, to the largest extent viruses associated with wild birds, especially those that occupy wetlands and in particular waterfowl (Alexander 2000b; Olsen et al. 2006). It is in waterfowl, and particularly mallards, that the largest genetic and antigenic variation of IAVs occurs (Fig. 9.1). In addition to wild birds and seasonal influenza in humans (Rambaut et al. 2008b), IAVs also circulate in pigs (Vincent et al. 2014), horses (Daly et al. 2011), marine mammals (Groth et al. 2014), bats (Wu et al. 2014b) and domestic birds (Olsen et al. 2006; Webster et al. 1992; Alexander 2000b). These viruses are subtyped based on the two surface proteins hemagglutinin and neuraminidase and are further classified as either highly pathogenic (HPIAV) or low pathogenic (LPIAV) based on their virulence in poultry (see Boxes 9.1 and 9.2).
Host range and transmission of IAV. The wild bird reservoir comprises of waterfowl and gulls (dark grey), with direct spillover to other avian species such as passerines and poultry. H5N1, which is amplified in poultry, has subsequently spilled over to wild bird species, mammalian hosts such as cats and dogs and humans. The relationship between bats and other host groups is unknown. Solid lines represent known routes of transmission, dashed lines are infrequent routes of transmission, and semicircles demonstrate circulation of IAV in that host group [Reproduced with permission, Wille (2015) LNU PRESS]
Box 9.1 Influenza Classification and Structure
Influenza viruses belong to the family Orthomyxoviridae (Kawaoka et al. 2005) and are divided into influenza A, influenza B and influenza C viruses. This division is based upon antigenic properties of the nucleocapsid (NP) and matrix (M) proteins and structural variations (Webster and Kawaoka 1988). Wild birds are naturally infected only with influenza A viruses (IAV) (Webster et al. 1992) The IAV virion is enveloped and spherical or pleiomorphic in shape with an approximate diameter of 120 nm (Webster et al. 1992). The IAVs are further classified based on two surface glycoproteins: hemagglutinin (HA) and neuraminidase (NA) which mediate entry and exit from the host cells, respectively. There are 18 HA and 9 NA forms, of which 16 HA subtypes are present in birds (Wu et al. 2014b; Olsen et al. 2006). These HA and NA subtypes can occur in 144 different combinations, such as H5N1 or H1N1.
The genome consists of eight segments of unlinked, negative-sense, single-stranded RNA: PB2 (polymerase basic protein 2), PB1 (polymerase basic protein 1), PA (polymerase acidic protein), HA, NP, NA, M and NS (nonstructural protein) (Kawaoka et al. 2005; Webster et al. 1992). These segments encode for ten core proteins, where the M and NS encode two proteins, and several auxiliary proteins (Webster et al. 1992). The different proteins have functions in entry (HA, M2), RNA replication (PB2, PB1, PA, NP), packaging (M1, NS2), exit from the host cells (NA, M1) and immune system evasion (NS, HA, NA) (Webster et al. 1992; Samji 2009).
Due to the segmented nature of the genome, these viruses are able to dramatically change their genotype (and phenotype) through reassortment. Following coinfection the resulting progeny could be any one of 256 possible combinations of the parental genotypes due to the process of virion packaging (Steel and Lowen 2014). Due to the error-prone RNA-dependent RNA polymerase that lacks proofreading ability, these viruses have a high mutation rate (3.4 × 10−3 sub/site/year) (Chen and Holmes 2006), which is about a million times that of vertebrates (Pybus and Rambaut 2009). This rapid rate of change allows for the continued immune evasion of antigenically important segments, such as the HA and NA.
Box 9.2 Determinants of Pathogenicity of Avian IAV
Avian IAVs are categorised into two groups: low pathogenic influenza A viruses (LPIAVs) and highly pathogenic influenza A viruses (HPIAVs). The pathogenicity trait is based on virulence of the virus in chickens and is an important consideration in prevention, control and eradication strategies in commercial fowl (Swayne and Suarez 2000). Wild birds infected with LPIAV generally show no clinical signs of infection (Olsen et al. 2006; Webster et al. 1992). However, it has been demonstrated that LPIAV infections may induce fever (Jourdain et al. 2010) and affect body mass and migratory ability (van Gils et al. 2007; Latorre-Margalef et al. 2009b; Jourdain et al. 2010), but overall the effects of LPIAV infection on wild birds are still poorly understood. In birds, LPIAVs preferentially infect the epithelium of the lower gastrointestinal tract and are shed predominantly through the feces (Webster et al. 1976, 1978; Slemons and Easterday 1978; Engering et al. 2013; Daoust et al. 2011). These viruses are thought to be transmitted mainly by faecal-oral route through bird-bird contact (Webster et al. 1992) and water-borne transmission (Webster et al. 1992; Roche et al. 2009).
In contrast, HPIAV preferentially infects the epithelium of the respiratory tract, including the trachea, lungs and air sacs (Bröjer et al. 2009; Keawcharoen et al. 2008; Worobey et al. 2014). However, lesions associated with HPIAV have been found throughout birds; these viruses are organ promiscuous (Bröjer et al. 2009) As a result, HPIAV infection normally results in significant morbidity and mortality of the infected bird host (Webster and Rott 1987; Alexander 2007). Mechanistically, the switch from LPIAV to HPIAV follows changes in the HA protein. The hemagglutinin protein is produced as a precursor, HA0, which is cleaved into HA1 and HA2 during virus maturation by host tissue-restricted proteases. The introduction of basic amino acid residues to the cleavage site allows for increased HA cleavability by more ubiquitous proteases, which, in turn, allows for enhanced replication outside the gastrointestinal tract (Alexander 2000a). The subtypes H5 and H7 have accounted for most HPIAV isolations in wild birds (Alexander 2007; Olsen et al. 2006). The switch from low to high pathogenicity forms occurs most often after the introduction of these LPIAV H5 and H7 into poultry (Alexander 2000b), and has never been documented in wild bird hosts (Alexander 2000b, 2007). HPIAV has been isolated predominantly from domestic gallinaceous birds (chickens, turkeys, quail) (Alexander 2000b; Perkins and Swayne 2001; Wobeser 1992; Chen et al. 2005), but spillover outbreaks have occurred.
Despite the broad host range, wild birds are the natural reservoir for LPIAV and exhibit no clinical symptoms of infection. Within this reservoir, LPIAVs have been isolated from at least 105 species in 26 different families, though this number has undoubtedly increased since the last substantial reviews of bird hosts in 2006 and 2007 (Olsen et al. 2006; Stallknecht and Brown 2007). However, all bird species are not equally permissive hosts, and different groups play different roles in the epidemiology and maintenance of IAV. Viruses are most frequently detected in Anseriformes (ducks, geese, swans), where H1–H12 are routinely detected (Munster et al. 2007; Alexander 2007; Olsen et al. 2006). Some Charadriiformes (shorebirds, gulls) are also important: most notable are gulls, which are the natural reservoir for H13 and H16 viruses and in which other subtypes are infrequently detected (Wille et al. 2011a; Arnal et al. 2014). These two subtypes are restricted to gulls, and ducks are not permissive to infection with H13 (Brown et al. 2012) or H16 (Fereidouni et al. 2014). Shorebirds play an interesting role, in that IAVs appear to be rare in this group, except in some species such as ruddy turnstone (Arenaria interpres) and red knot (Calidris canutus) in one geographic location: Delaware Bay, USA. This site is recognised as an important stopover location, and many different IAV subtypes are detected in spring-staging shorebirds; however, it is believed that shorebirds amplify LPIAV circulating in local waterfowl and the dynamics are the result of a unique ecological event: the spawning of horseshoe crabs (Limulus polyphemus) (Pearce et al. 2009; Winker et al. 2008; Maxted et al. 2012; Krauss et al. 2010). Other bird orders are believed to be spillover hosts, including the species-rich Passeriformes (Slusher et al. 2014) (Fig. 9.1). Within the Anseriformes, dabbling ducks, and particularly mallards, have accounted for most LPIAV isolations globally (Olsen et al. 2006). This may in part be due to sampling bias (Hoye et al. 2010), but it is highly likely that dabbling ducks, such as mallards, do actually have higher infection rates than other species.
The importance of waterfowl IAVs, and more recently poultry-adapted IAVs, in the context of emerging disease, is when they occasionally transmit to other species, particularly to mammals (humans, pigs, horses). Genetic barriers between host groups limit the free transmission of IAVs; however, spillovers do occur. These spillover events may result in isolated outbreaks with little or no onward transmission, such as spillover of HPIAV H5N1 to dogs (Songserm et al. 2006b), cats (Songserm et al. 2006a) and tigers (Mushtaq et al. 2008) or LPIAV H10 into seals (Bodewes et al. 2015; Zohari et al. 2014). The continued spillover of LPIAV H7N9 to humans (Gao et al. 2013; Kageyama et al. 2013), and subsequent adaptation to mammalian hosts, is of further concern. Rather than the spillover of entirely avian viruses, it is the incorporation of avian or swine gene segments that is of high concern as at least three major human pandemics of IAV were caused by viruses containing gene segments of avian origin (Lindstrom et al. 2004; Rabadan et al. 2006; Scholtissek et al. 1978; Taubenberger et al. 2005), and swine viruses played a role in the most recent H1N1 pandemic. Indeed, in an analysis of cross species transmission, wild birds, domestic birds and swine showed the highest connectivity, and further, swine and wild birds were the dominant species for global virus delivery (Ren et al. 2016). Further, Worobey et al. (2014) proposed that the Western Hemisphere panzootic of equine influenza in 1872–1873 may have resulted in the introduction of equine origin segments into human and avian IAV, particularly of the internal genes into avian IAV lineages. This combined with the first records of highly pathogenic avian influenza in poultry, which coincide with the transition to industrial animal production, may have been imperative in the successful emergence of novel avian viruses (Worobey et al. 2014). As such, reassortment is the driving factor in the ability of IAV to successfully emerge in multiple host species and remerge in populations.
Phylogeography of IAV in birds is shaped by host species movement and migration patterns.
Many dabbling duck species are migratory, or partial migrants, in the Northern Hemisphere, generally displaying a higher propensity for migration the further north the breeding distribution is located. In tropical regions, ducks are either resident or migrate in relation to rain and dry seasons, sometimes with irruptive movements. However, compared to other bird groups like gulls, terns or shorebirds, they show less long-distance migrations across substantial geographic barriers, such as oceans or deserts. As a result, they tend to migrate within the Old World (Europe, Africa and Asia) and the New World (North and South America) (Olsen et al. 2006). Due to the geographically segregated nature of their waterfowl hosts, avian IAVs can be divided into two main phylogenetic clades: Eurasian and North American (Olsen et al. 2006). More recently, it has been proposed that there may be a distinct IAV lineage in South America as well (Pereda et al. 2008; Nelson et al. 2016), perhaps reflected by limited waterfowl migration across the Gulf of Mexico or the Isthmus of Panama. Indeed, more recent work in blue-winged teals (Anas discors) in two different parts of their migratory routes, in the southern USA and Guatemala, has demonstrated viral phylogenetic signal from North America, rather than from South America (Ramey et al. 2014; Gonzalez-Reiche et al. 2012).
This pattern of hemispheric signal due to independently evolving major lineages is conserved across all eight RNA segments of the IAV genome; however, due to occasional introductions and subsequent competitive exclusion, some of these broad geographic lineages are replaced (Bahl et al. 2009, 2013). Within waterfowl hosts, it is extremely rare to find a virus with a geographic mosaic of segment origin. Winker and Gibson quantified avian movement between Asia and Alaska and demonstrated a large influx of birds between these continents (Winker and Gibson 2010). More targeted work in species such as northern pintails (Anas acuta), a species that breeds on both sides of the Bering Strait, has demonstrated movement of viral segments from Asia into North America (Koehler et al. 2008; Ramey et al. 2010). However, despite sharing habitats with these pintails, detection of IAV with differing geographic origins within Alaskan mallards is infrequent (Pearce et al. 2011), suggesting that there may be some type of host species barrier or fitness consequences for these mosaic viruses. It had been hypothesised that this concept of a natural host species barrier prevented Asian viruses from entering North America. However, more recently, it has been proposed that wild birds migrating between Asia and Alaska were the conduit for the introduction of highly pathogenic H5N8 to North America (highly pathogenic IAV is further discussed in Box 9.2 and Sect. 9.3.2.5) (Ramey et al. 2016; Lee et al. 2015). Unlike waterfowl, geographic mosaic viruses are more common in gulls (Wille et al. 2011a, b; Huang et al. 2014b; Dusek et al. 2014), which is in part driven by gulls having different migration and movement patterns as compared to ducks. For instance, great black-backed gulls (Larus marinus) banded in eastern Canada have been recorded in Western Europe (Wille et al. 2011b). Similarly, studies of common murres (Uria aalge), which may interact with other seabirds and gulls in overwintering areas, have detected virus genomes with geographic mosaicism (Huang et al. 2014a; Lang et al. 2016). Genetically, there does not seem to be host species segregation in avian IAV, with the exception of gulls, wherein there are gull-specific lineages for the NA, M, NP and NS segments and the HA subtypes H13 and H16 are gull specific (Chen and Holmes 2009; Wille et al. 2011a).
The large genetic diversity of IAVs is a result of two mechanisms: genetic drift and genetic shift. Genetic drift occurs due to an error-prone RNA-dependant RNA polymerase, which lacks proofreading ability (Gething et al. 1980; Both et al. 1983; Webster et al. 1992). An early concept in IAV evolution was that avian IAVs are in “evolutionary stasis” in that the evolutionary arms race between host and virus is less intense in avian systems resulting in little selective requirement to repeatedly fix amino acid changes that evade the immune response (Chen and Holmes 2006; Suarez 2000). This hypothesis has been refuted using genetic studies demonstrating high rates of mutation due to genetic drift. Avian IAVs have been demonstrated to have rapid rates of evolutionary change, characterised by accumulations of synonymous and non-synonymous mutations (Chen and Holmes 2006, 2010; Bahl et al. 2009). A synonymous mutation is one which changes the nucleic acid sequence without changing the amino acid sequence of the encoded protein. Non-synonymous nucleic acid mutations do change the amino acid sequence. The rate of mutations varies across segments, with an average rate of 3.41 × 10−3 substitutions/site/year. To put this in context, the rate of IAV mutation is a million times greater than that of vertebrate genomic DNA, and this allows for the rapid adaptation of these viruses to new environments (Pybus and Rambaut 2009). A result is that there are a number of forward evolving lineages for all RNA segments.
The second mechanism through which IAV can diversify is genetic shift, which occurs due to coinfection and reassortment. Reassortment occurs due to the unlinked nature of the eight RNA segments, and thus, if a cell is infected by more than one virus, the progeny virions may contain various combinations of segments from the different parental viruses (Webster et al. 1992; Gething et al. 1980). Thus, given a coinfection with two IAVs, each with 8 segments, 256 different genetic progenies are possible, generating significant viral diversity (Ma et al. 2016). Due to the frequent reassortment in avian IAVs, virus genotypes, or genome constellations, are rarely isolated across consecutive days at the same location (Dugan et al. 2008). Given this, it is unsurprising that across an autumn season, over 50% of viruses from mallards are reassorted, across a number of different subtypes (Wille et al. 2013). Furthermore, this is likely driven by seasonal dynamics of subtype presence and virus load in the population (Wille et al. 2013). Thus, IAVs do not circulate as fixed genome constellations, but rather as transient constellations that rapidly change, even within the same host species, location and time period (see Box 9.3).
Box 9.3 Influenza A Virus Evolutionary Genetics
Genetic drift and shift do not necessarily correspond to antigenic change or change in phenotype. However, given change in phenotype, the progeny viruses may have a selective advantage due to host immune system evasion. Studies of human IAV H3N2 have demonstrated that genetic drift is a gradual and continuous process, resulting in a ladder-shaped phylogeny (Rambaut et al. 2008a). Antigenic shift, however, is more punctuated in that the accumulation of a number of mutations at specific positions will result in viruses occupying a new phenotype (Koel et al. 2013; Smith et al. 2004). Our comparatively less knowledge regarding antigenic change and inter-lineage evolution in avian IAVs is partly due to large concurrent genetic variation and insufficient sampling but also compounded by a possible long-term tenacity of viruses in the abiotic environment where “old” viruses have been hypothesised to reappear in the population of birds after some time (Roche et al. 2014).
Genetic drift and genetic shift allow for IAV to rapidly diversify; however, current genetic structure of IAV is due to an interplay between diversification and selective sweeps in the population. Viruses with specific genome constellations that attain a much higher fitness will rapidly increase in frequency. These constellations may drive competing lineages to extinction and may be driven to fixation, thus eliminating circulating diversity—known as a selective sweep. Although it is only the antigenic segments that may be selected for, as these are the ones that interact directly with the immune system, the other segments in that successful constellation will be “carried along”, demonstrating a “hitchhiking” mechanism (Chen and Holmes 2010). As a result, there will be a selective sweep across not only antigenic segments but all segments of IAV.
Despite a proposed ancient co-evolution of birds and IAVs, dating of current lineages suggests these are of recent origin. The time of origin of the circulating PB2, PB1, PA, NP and M segments is only approximately 100–130 years ago. The most recent common ancestor for the more divergent HA, NA and NS segments is more ancient; however, intra-subtype radiation occurred more recently as well (Chen and Holmes 2006; Worobey et al. 2014). Coincidentally, during that time period when the first descriptions of HPIAV in domestic chickens occurred, there was a transition to more intensive chicken farming. Additionally, the time period 1872–1873 corresponds with a severe panzootic of equine influenza, coupled with reports of influenza in domestic birds following local equine outbreaks (McDonald et al. 2009). Thus, it is hypothesised that these events resulted in a global sweep of avian IAV resulting in these shallow divergence times (Worobey et al. 2014). While this global sweep has had large implications in the genetic structure of IAV, numerous local sweeps have occurred as well, driven by the introduction of a novel segment or segments following reassortment (e.g. Bahl et al. 2009).
3.2.2 Dynamics of IAV in Mallards: All Birds Are Not Equal
Mallard has high IAV prevalence across years and locations, and the largest number of viruses has been isolated from this species and with a high diversity of subtypes (Olsen et al. 2006, 2014). This species is also a dominant component in species composition of many IAV surveillance studies. A review by Olsen et al. (2006) demonstrated that nearly 50% of all waterfowl samples analysed for IAV were from mallard, with a global viral prevalence of 12.9%. The number of collected samples has risen dramatically since the review; a search of the Influenza Research Database (IRD; http://www.fludb.org) indicates 64,194 samples have been collected from mallards with 3271 HA sequences generated. In our own study site in southeast Sweden, 22,229 cloacal/faecal samples were collected in 2002–2009, generating 1081 isolated IAVs across 74 HA-NA subtypes (Latorre-Margalef et al. 2014) (Fig. 9.3b).
The prevalence of IAV in mallard follows a seasonal pattern, whereby it is low during the late winter, spring and summer, followed by a peak in viral prevalence during the autumn migration (Latorre-Margalef et al. 2014; Wilcox et al. 2011; Ito et al. 1995; Hatchette et al. 2004; Olsen et al. 2006). This pattern has been observed at a number of study sites across the Northern Hemisphere, including Sweden (Latorre-Margalef et al. 2014), the Netherlands (Munster et al. 2007; van Dijk et al. 2014), Canada (Alberta; Hatchette et al. 2004; Sharp et al. 1997), the USA (Minnesota; Wilcox et al. 2011), California (Hill et al. 2012) and Alaska (Ip et al. 2008; Runstadler et al. 2007), and prevalence can be up to 30% during the autumnal peak.
This prevalence pattern is largely driven by the ecology of the waterfowl hosts. In the autumn months, widely dispersed breeding individuals congregate during migration, which increases host density. Further, during the summer months, breeding has led to the production of young, which are immunologically naïve. The high turnover rate of mallards results in a substantial recruitment of immunologically naïve individuals into the population (Munster and Fouchier 2009; Bentz 1985). Not only do hatch-year birds account for more infections than all other age classes (Wilcox et al. 2011; Ip et al. 2008; Webster et al. 1992), but it also has been demonstrated that young birds and migrants are important drivers in IAV dynamics (van Dijk et al. 2014; Avril et al. 2016).
Compounded with an increase in immunologically naïve individuals, there is a decline in anti-IAV antibodies in second-year mallards during the summer months, suggesting a decrease in general herd immunity during this period allowing for reinfections with IAV the next autumn (Tolf et al. 2013a). Thus, it is a combination of mallard phenology, ecology and biology that are drivers for the seasonal pattern of IAV prevalence (Fig. 9.2).
(a) Seasonal dynamics of IAV in mallard is influenced by an input of immunologically naïve individuals and a decrease in immunity. (b) Seasonal prevalence of IAV, also illustrated by the second concentric circle of the schematic. (c) Number and proportion of newly ringed mallards at Ottenby demonstrating an increase in young or newly ringed individuals in the summer prior to the prevalence peak. (d) Seasonal levels of anti-NP antibodies of second-year birds living in a duck trap demonstrating individual variation in retention of immunity, long-term immunity following infection in the previous autumn and a marked drop in antibodies during the summer months followed by an increase following reinfection in the autumn [Panel A is modified from Latorre-Margalef (2012) LNU Press, Panels B and C were reproduced with permission from the Proceedings of the Royal Society B, and Panel D was modified from Tolf et al. (2013a) PLoS One]
Within the autumnal prevalence peak, there are many different HA-NA subtypes co-circulating. To date, 102 of the possible 144 HA-NA subtype combinations have been detected in wild birds, globally (Olson et al. 2014) (Fig. 9.3a). Mallards represent a substantial proportion of this figure, whereby 74 HA-NA subtype combinations have been detected in mallards from a Swedish study site alone (2002–2009), and most globally detected subtypes have been found in mallard and other Anseriformes elsewhere (Figs. 9.1 and 9.3). Within mallards, some IAV subtypes are very common, and others are either rare or absent. Common subtypes are usually isolated every year, such as H6 and H4 in Europe (Latorre-Margalef et al. 2014) (Fig. 9.3b) or H3 in North America (Bahl et al. 2013; Bahl et al. 2009; Wilcox et al. 2011). Some subtypes exhibit a more outbreak-like pattern, whereby they are common in some years and absent in others (Thangavel et al. 2011; Wilcox et al. 2011; Latorre-Margalef et al. 2014). Rare viruses may be isolated in low numbers every year or only sporadically. This overall pattern is observed across the Northern Hemisphere; however, small differences also occur between study sites and continents (Bahl et al. 2013; Latorre-Margalef et al. 2014; Olson et al. 2014). Furthermore, some HA-NA combinations are overrepresented, such as H4N6, H6N2 and H3N8, which are consistently isolated. Alternatively, some HA subtypes may be paired with any NA subtypes, suggesting fitness differences between HA-NA subtypes (Latorre-Margalef et al. 2014; Dugan et al. 2008). Surprisingly, when challenging ducks with combinations that are common (e.g. H3N8) compared to uncommon (e.g. H4N8), there appear to be no fitness differences such as virus load or duration of shedding (Lebarbenchon et al. 2012); thus, the mechanisms that drive subtype abundance patterns are still unknown.
Subtype diversity of IAV. (a) Global subtype distribution and detection across different avian host groups: Anseriformes, Charadriiformes, Procellariiformes, more than one order and poultry (Modified from Olson et al. 2014). (b) Subtype distribution and frequency in mallards utilising Ottenby Bird Observatory in Sweden as a stopover side, 2002–2009 (Modified from Latorre-Margalef et al. 2014)
Furthermore, some subtypes or group of subtypes appear early in the season, and others appear late in the season. This is attributed to HA subtype-specific immunity against specific subtypes (homosubtypic immunity) or closely related subtypes (heterosubtypic immunity) (Latorre-Margalef et al. 2013). For example, in our long-term monitoring of migratory mallards at a stopover site in SE Sweden, the first viruses to appear in the season are normally H3 class viruses, which include phylogenetically related H3, H4, H7 and H10 subtypes. This is in contrast to the later-arriving H1 class viruses (H1 clade (H1, H2, H5 and H6) and H9 clade (H8, H9 and H12)) (Latorre-Margalef et al. 2014). This is a relatively new way of characterising IAV subtype dynamics and therefore has not been assessed at other study sites.
Most field studies conducted on LPIAV in mallard focus on describing population-level parameters, such as virus prevalence or seroprevalence. Although important, population-level data can mask processes occurring at the individual level and fail to acknowledge individual variation in infection patterns. The biggest hurdle for conducting individual-level disease ecology studies is to follow individuals and their disease states over time. One approach used by Tolf et al. (2013a) was to introduce immunologically naïve commercially reared mallards in a duck trap used for attracting wild birds. These ducks became naturally infected by their wild conspecifics as they were sharing water and separated only by some mesh, and by daily sampling of these birds, it was possible to create individual disease histories of 1.5 years of length. Although these birds shared overall trends, there were considerable differences between individuals in which they were infected with LPIAV subtypes, coinfection patterns, lengths of shedding, clearance of infection and immune responses (Fig. 9.4) (Tolf et al. 2013a). Another approach is to use data from wild birds that are captured repeatedly over time. The resulting disease histories can be analysed by capture-mark-recapture (CMR) modelling techniques to estimate how infection parameters are affected by host categories (such as age and sex) and seasonal factors. Using multistate-CMR models on 3500 individual mallards across seven autumn seasons, Avril et al. (2016) demonstrated individual-level differences in both infection force and recovery rate. Specifically, for most years, prevalence and risk of LPIAV infection peaked at a single time during the autumn migration season, but the timing, shape and intensity of the infection curve showed strong annual heterogeneity. In contrast, the seasonal pattern of recovery rate only varied in intensity across years. Adults and juveniles displayed similar seasonal patterns of infection and recovery each year. However, juveniles experienced twice the risk of becoming infected as compared to adults, whereas recovery rates were similar across age categories (Avril et al. 2016).
Ten mallards residing in a duck trap were sampled daily across autumn 2009. Different individuals show different patterns of infection, despite following the expected seasonal trend. Individuals are plotted on the y-axis. White space indicates birds were negative for influenza, and colours refer to different HA subtypes with the exception of grey, which are samples positive by real-time PCR but negative by culture and therefore not subtyped (Modified from Wille et al. 2013, Virology)
3.2.3 Mallard Immunity to Influenza A Virus
Most microorganisms are immediately and non-specifically detected and cleared by the innate immune system, and it may alone succeed in repelling the pathogen while allowing time for the adaptive immune response to be mounted. Infection by IAV in ducks is initially combatted by components of the innate immune such as interferon-induced proteins. The adaptive immunity then develops neutralising antibodies, both subtype specific and those to conserved epitopes across subtypes (Magor 2011; Vanderven et al. 2012; Lundqvist et al. 2006). Taken together, this means that IAV infection in mallards is acute and of short duration—the average length of infection is 1 week, depending on host type, age and previous infection history. The complexity of the mallard immune system and response is still being disentangled. Following infection, it has been demonstrated that RIG-I (retinoic acid-inducible gene 1) is highly upregulated at the site of infection: the gastrointestinal tract or lungs of ducks infected with LPIAV and HPIAV, respectively (Barber et al. 2008; Vanderven et al. 2012). The RIG-I gene is absent in chickens and may explain why chickens display severe morbidity and mortality following infection, whereas mallards may display no clinical signs of disease (Barber et al. 2008; Vanderven et al. 2012). Other important innate immune genes are effectors of the interferon (IFN) pathways. Interestingly, the major histocompatibility complex (MHC), a part of the acquired immune response, appears to be important both early and late in IAV infection (Vanderven et al. 2012).
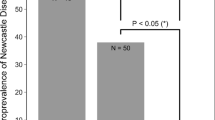
Despite combatting LPIAV infection rapidly, ducks may have poor long-term immune memory (Magor 2011), illustrated by a pattern of seroconversion and seroreversion (Tolf et al. 2013a), hypothesised to be due to the structure of the immunoglobulins, such as the translocation of the IgA (Magor 2011; Magor et al. 1998, 1999) and a truncation of the IgY (Warr et al. 1995) which appear to affect antibody functionality. Truncated IgY is able to neutralise viruses but is not involved in agglutination, complement fixation or opsonisation (Lundqvist et al. 2006). However, infection experiments (Fereidouni et al. 2010) and natural experimental infections (Tolf et al. 2013a) have demonstrated the presence of anti-NP antibodies, which are not neutralising, for months following infection. In long-lived species, such as shearwater, geese or swans, antibodies may be long-lived—antibodies against a Newcastle disease virus were detectable for a number of years in Cory’s shearwater (Calonectris borealis) (Ramos et al. 2014), and in swans and geese, antibody prevalence increases with age, suggesting long-term antibody retention and accumulation with age (Hill et al. 2016).
Hill et al. further demonstrated that the breadth of antibody response increases with age, that is, individuals have neutralising antibodies against a larger number of HA subtypes with age (Hill et al. 2016). In chickens, protection against IAV is primarily through antibodies directed at the HA (Kapczynski and Swayne 2009), which is likely also true in mallards. Antibodies directed against NA or other proteins may contribute to clearance of infection (e.g. Nayak et al. 2010), but it is unclear. As previously mentioned, both homo- and heterosubtypic immunity develop following natural infections, where individuals infected with a particular subtype are unlikely to be reinfected with that same HA subtype later in the season (homosubtypic immunity) and across seasons (Tolf et al. 2013a; Latorre-Margalef et al. 2013). That is, ducks infected with H3 viruses should not be reinfected with H3 viruses. Homosubtypic immunity, however, is not always complete, and a field study utilising vaccines demonstrated the escape of an H3 virus from H3 neutralising antibodies which was hypothesised to be due to antigenic shift in the field viruses (Wille et al. 2016). This phenomenon is also seen in escape of HPIAV H5 viruses from the H5 vaccine in birds. Furthermore, partial or complete protection is apparent when reinfected with a closely related HA subtype (heterosubtypic immunity). Therefore, the duck previously infected with H3 could be protected, and thus not infected, by the closely related H4 virus. Homo- and heterosubtypic immunity have largely been explored using experimental infections, and many have been done so in the context of vaccine development and cross-protection against highly pathogenic H5 and H7 viruses (Costa et al. 2010, 2011; Fereidouni et al. 2009, 2010). But, more recently, studies have begun to explore protection and immunity patterns in low pathogenic infections, for example, Segovia et al. (2017) which investigated H3N8, H4N6, H10N7 and H14N5 infections in a balanced design (Segovia et al. 2017). Latorre-Margalef et al. (2016a, b), which assessed protection of H3 antibodies against an array of other virus subtypes, showed that the degree of protection was correlated with phylogenetic relatedness between viruses, where highest protection was induced to closely related HAs (Latorre-Margalef et al. 2016a, b). This acquired immunity shapes the dynamics of many diseases and is likely a driver for the continuing divergence of HA types. Given these findings, one can hypothesise that it is this immunity that drives the order and patterns of subtypes that occur in a population of birds across an autumn season. However, this premise warrants further study, especially tests of how HA-specific immunity and cross immunity affect future infection probability and virus load.
While there is great interest in IAV ecoimmunology, we are still largely using proxies for the mallard immune response, with a focus on the acquired immune response. In order to better understand host response to infection, continued work assessing the response of the innate response is imperative, as this response is coupled to the acquired response and may explain some of the patterns we see at this level.
3.2.4 Impact of LPIAV Infection on Hosts and Host Ecology
There is limited and contentious knowledge regarding the effect of LPIAV infection on wild birds, on short- and long-term impacts on host fitness, either at the individual bird level or bird population level. The current dogma is that birds, especially dabbling ducks, infected with LPIAV exhibit no clinical disease signs, despite being infected and reinfected with a virus and shedding these viruses at high viral loads in the gastrointestinal tract. These viruses replicate in the surface epithelium of the respiratory tract and gastrointestinal tract, and gross lesions are absent at the site of infection in natural infections (Kuiken 2013) (Box 9.2). Furthermore, there does not appear to be any increase (or decrease) in immune parameters of mallards naturally infected with LPIAV (van Dijk et al. 2015b). Granted there is a limited physiological response of individuals, this may still translate into short-term ecological effects as infections tend to be acute and short. Wild birds can experience physiological stress as a result of limited nutritional resources and variable energy expenditure during the year, which could have an effect on the course of disease within the host and therefore the host population. Interestingly, poor body condition due to food limitation in mallards in the context of IAV infection has indicated limited viral shedding compared to individuals in good condition (Arsnoe et al. 2011). Latorre-Margalef et al. (2009b) demonstrated a negative impact of LPIAV infection on body mass, and the amount of virus shed by infected juveniles was negatively correlated with body mass. This has been countered, wherein it is unclear if LPIAVs affect the body mass of individuals or whether birds in poor physical condition are more susceptible to acquiring infection (Flint and Franson 2009; Latorre-Margalef et al. 2009a). In a study of white-fronted geese (Anser albifrons), individuals with a lower body weight had a higher probability of infection but only for 1 of 4 years (Kleijn et al. 2010). In turn, during a study on Bewick’s swans (Cygnus bewickii), it was found that birds experiencing their first infection (naïve-infected) had a reduced foraging rate but had similar body stores to reinfected and uninfected individuals (Hoye et al. 2016). This study reflects the reduced refuelling and feeding rates detected in an earlier study (van Gils et al. 2007).
Latorre-Margalef et al. (2009b) further found no effects of overall staging time or the speed and distance of subsequent migration. van Dijk et al. (2015a) found a weak negative association between LPIAV infection in mallard and regional movements (>100 m) on the final days of tracking, being exacerbated by poor weather conditions (van Dijk et al. 2015a), but a recent tracking study in Sweden found no differences in activity or movement between infected and uninfected mallards during stopover in autumn (Bengtsson et al. 2016). In naturally infected Bewick’s swans, which demonstrated reduced refuelling and feeding rates, the birds also had delayed and protracted migration distances as when infected with LPIAV; however, the sample size of this study was only two infected birds (van Gils et al. 2007). This trend was not observed when applying a more experimental set-up wherein birds were infected and released (Hoye 2011). Interestingly, a follow-up study on Bewick’s swans detected a potential difference in survival, where naïve-infected swans were unlikely to be resighted 1 year after infection, compared to uninfected or reinfected individuals (Hoye et al. 2016). This study also illustrates [a difference in response] between individuals infected for the first time and those uninfected and reinfected, whereby [birds that have been infected] and reinfected [have similar responses to birds that have never been infected] (Hoye et al. 2016). This is perhaps not surprising as immunologically naïve individuals have a much higher risk of infection (Avril et al. 2016).
Despite limited physiological signs of infection, it has been hypothesised that LPIAV infection may be affecting digestive tract functioning. Wild birds delicately balance energy intake and energy output, and decreased gastrointestinal functioning could translate into reduced body mass, delayed staging or decreased movements of individuals (Kuiken 2013). As of yet, there are few studies using natural systems, due to the difficulty in carrying out such experiments and disentangling all the confounding factors during data analysis. Experimental infections may provide insight; however, these studies rarely reflect natural conditions, and the results are dependent upon mode of inoculation, strains and conditions (Kuiken 2013). Low virulence and limited clinical signs have been interpreted as a long-standing co-evolutionary relationship between IAV and the host (van Dijk et al. 2015b), but further research addressing this is warranted.
3.2.5 LPIAV, HPIAV and the Interface with Poultry
In the sections above, we have mainly addressed wild birds as carriers of low pathogenic viruses (for extended definition, see Box 9.2). However, viruses with a highly pathogenic phenotype can be detected, either as spillover infections or in sustained transmission among wild waterfowl. Actually, the very first record of IAV in wild birds was an outbreak in common terns (Sterna hirundo) in South Africa, 1961, resulting in the mortality of at least 1300 individuals (Becker 1966). This record remains unusual as it is the only recorded case of an outbreak of HPIAV in wild birds with no direct link to outbreaks in poultry.
The HPIAV H5N1, colloquially referred to as “bird flu”, was first identified in 1996; however, it wasn’t until 2005 that it resulted in the mass mortality of wild and domestic birds alike (Gauthier-Clerc et al. 2007; Feare 2010; Chen et al. 2005). It has since spread to countries in Asia, the Middle East, Africa and Europe, resulting in the culling of 400 billion chickens, turkeys and ducks and over 600 human cases (FAO 2012). Despite many years of research and vaccine development, this virus continues to cause outbreaks in Asia and Africa (FAO 2012). In November and December 2014, there were new incursions of HPIAV H5 into Europe and North America, the latter of which is a geographic range expansion. A novel HPIAV H5N8 resulted in the culling of poultry in Asia, Europe and North America. This strain was first reported in Chinese duck farms in 2010 (Wu et al. 2014a) and was detected in both poultry and wild birds in Korea, following an outbreak in 2014 (Lee et al. 2014). North America had not previously been affected by HPIAV H5N1, and the proposed conduit for entrance of this virus into North America is Beringia or from Asia into Alaska with migrating wild birds (Lee et al. 2015; Ramey et al. 2016). Unlike HPIAV H5N1, the HPIAV H5N8 doesn’t appear to cause widespread morbidity or mortality in wild birds; hence, it entered North America and Europe virtually undetected in wild birds; that is, following mortality events in poultry, it was detected in wild birds from surveillance studies that were retrospectively screened (e.g. Ramey et al. 2016). Further, in North America, there is evidence that HPIAV H5N8 has reassorted with low pathogenic avian viruses resulting in HPIAV H5N1, H5N2 and H5N8 (e.g. Pasick et al. 2015). Similar to HPIAV H5N1, this virus has been detected in wild ducks in Asia, Europe and North America, suggesting wild birds as contributors in the long-distance dispersal (Gauthier-Clerc et al. 2007; Feare 2010; European Food Safety Authority 2014; Verhagen et al. 2015; Lee et al. 2015). Intriguingly, H5N8 seemed to disappear from North America following massive expansion in 2014 prompting questions about the role of wild birds in perpetuating this virus (Krauss et al. 2016). However, there have been severe outbreaks of H5N8 in 2016/2017 in North America and further across the globe.
The HPIAV infection experiments conducted on waterfowl have shown large variation in disease severity depending on the host species (e.g. Perkins and Swayne 2001; Perkins and Swayne 2002; Ellis et al. 2004; Keawcharoen et al. 2008; Brown et al. 2006, 2008; Pasick et al. 2007; Liu et al. 2005). Generally, dabbling ducks show fewer and less severe symptoms—and are sometimes asymptomatic despite shedding virus—than other duck species such as diving ducks (Bröjer et al. 2009; Pantin-Jackwood and Swayne 2007). This may be explained by intrinsic factors of the host such as the composition of immune branches and type/severity of the immune response. For example, RIG-I seems to be important in clearing IAV infection and is present in mallard but absent in chickens (Barber et al. 2008). Difference in response may also be partially explained by previous exposure to LPIAV, which reduces disease symptoms. For example, in an experimental study, birds that were first exposed to LPIAV had a less severe response to HPIAV after being reinfected (Fereidouni et al. 2009). The intrinsic features of wild birds that result in differing levels of infection are largely unknown, which is perhaps the result of our limited understanding of ducks (and wild bird) immune responses. Furthermore, despite numerous surveillance schemes, we are unable to predict the emergence and expansion of highly pathogenic IAV, as clearly illustrated by the recent emergence and range expansion of HPIAV H5N8.
4 Future Directions
What can we learn from the mallard in terms of ecology and evolution of disease? Not surprisingly, the first thing to note is how little we yet know of diseases in wildlife, especially for those diseases that can infect multiple host species and display large strain/antigenic variation. With the exception of IAV, the current knowledge on basic parameters such as host range, prevalence and distribution is sketchy at most for avian diseases. Interest in avian pathogens has primarily been driven by unexpected events, such as the introduction of West Nile virus in the USA or the spread of HPIAV H5N1 in Europe, resulting with intense surveillance activity for a few years and then winding down again with the entrance of another attention-grabbing disease on the scene. A more systematic sampling approach is needed, preferably representing a long-term focus coupled with large-scale efforts to study pathogens from their wild hosts. Fortunately, with the development of molecular methods and decreasing sequencing costs, we are better equipped for conducting these types of studies, and it is expected that the available information will increase substantially the coming years. Although important, molecular detection is the starting point, not the goal; in order to address ecological and evolutionary questions more accurately, they need to be complemented with efforts to isolate and characterise pathogens (Latorre-Margalef et al. 2016a, b; McClintock et al. 2010). This allows for functional analyses of pathogenicity and virulence, either in vitro or in animal models.
For IAV, long-term monitoring studies are available representing Europe and North America, and studies are emerging from Asia, Africa and South America, too. Collectively, these studies have provided genome data across the range of the virus enabling studies of evolutionary questions. However, for phylodynamic studies, even these large datasets are often insufficient as the global diversity of IAV is so large, leading to undersampling issues and, often, limited and biased spatial-temporal resolution. We do, however, have a basic understanding on the natural dynamics of LPIAV in wild mallards, including how virus prevalence varies between age classes and over time. Although most of the current literature focuses on host population-level data, an increasing trend for analyses conducted at the individual level is evident. This includes approaches to study movements and stopover behaviour in relation to infection, as well as capturing individual-based epidemiological parameters of disease dynamics (e.g. Avril et al. 2016; Tolf et al. 2013a; Latorre-Margalef et al. 2014). These in-depth, long-term studies are extremely valuable, and the continuation of such series (although expensive and logistically challenging) will be an important part of future research.
Building on the advances made during the last 50 years, the IAV research field is well suited to combine ecology and epidemiology for disease studies. Of particular interest would be to use the mallard-IAV system, for which we have a lot of “baseline” data, and to focus more on physiology such as the effect of infection on hosts, ecoimmunology or the interplay between the immune response following infection and host life history traits—characterising the immune systems and general host immunological responses to infections—and the interplay between IAV and other members of the virome and microbiome, to illuminate interspecies transmission and reveal dynamics within the Anseriform reservoir beyond mallards or to tackle questions pertaining to basic epidemiological and disease ecology theory such as host range, resistance vs tolerance, etc. Surveillance and characterisation studies are imperative, however, as future advances almost certainly will hinge on multidisciplinary work.
References
Adrian WJ, Spraker TR, Davies RB (1978) Epornitics of aspergillosis in mallards (Anas platyrhynchos) in north central Colorado. J Wildl Dis 14(2):212–217
Alexander DJ (2000a) Newcastle disease and other avian paramyxoviruses. Rev Sci Tech OIE 19:443–462
Alexander DJ (2000b) A review of avian influenza in different bird species. Vet Microbiol 74:3–13
Alexander DJ (2007) An overview of the epidemiology of avian influenza. Vaccine 25:5637–5644
Alexander DJ, Banks J, Collins MS, Manvell RJ, Frost KM, Speidel EC, Aldous EW (1999) Antigenic and genetic characterisation of Newcastle disease viruses isolated from outbreaks in domestic fowl and turkeys in Great Britain during 1997. Vet Rec 145(15):417–421
Allison AB, Ballard JR, Tesh RB, Brown JD, Ruder MG, Keel MK, Munk BA, Mickley RM, Gibbs SE, Travassos da Rosa AP, Ellis JC, Ip HS, Shern-Bochsler VI, Rogers MB, Ghedin E, Holmes EC, Parrish CR, Dwyer C (2014) Cyclic avian mass mortality in the northeastern United States is associated with a novel orthomyxovirus. J Virol 89(2):1389–1403. doi:10.1128/JVI.02019-14
Anthony SJ, Epstein JH, Murray KA, Navarrete-Macias I, Zambrana-Torrelio CM, Solovyov A, Ojeda-Flores R, Arrigo NC, Islam A, Ali Khan S, Hosseini P, Bogich TL, Olival KJ, Sanchez-Leon MD, Karesh WB, Goldstein T, Luby SP, Morse SS, Mazet JA, Daszak P, Lipkin WI (2013) A strategy to estimate unknown viral diversity in mammals. mBio 4(5):e00598–e00513. doi:10.1128/mBio.00598-13
Arnal A, Vittecoq M, Pearce-Duvet J, Gauthier-Clerc M, Boulinier T, Jourdain E (2014) Laridae: a neglected reservoir that could play a major role in avian influenza virus epidemiological dynamics. Crit Rev Microbiol 41(4):508–519. doi:10.3109/1040841X.1042013.1870967
Arsnoe DM, Ip HS, Owen JC (2011) Influence of body condition on influenza A virus infection in mallard ducks: experimental infection data. PloS one 6(8):e22633. doi:10.1371/journal.pone.0022633
Avril A, Grosbois V, Latorre-Margalef N, Gaidet N, Tolf C, Olsen B, Waldenström J (2016) Capturing individual-level parameters of influenza A virus dynamics in wild ducks using multistate models. J Appl Ecol 53(4):1289–1297. doi:10.1111/1365-2664.12699
Bahl J, Vijaykrishna D, Holmes EC, Smith GJD, Guan Y (2009) Gene flow and competitive exclusion of avian influenza A virus in natual resevoir hosts. Virology 390:289–297
Bahl J, Krauss S, Kuhnert D, Fourment M, Raven G, Pryor SP, Niles LJ, Danner A, Walker D, Mendenhall IH, Su YC, Dugan VG, Halpin RA, Stockwell TB, Webby RJ, Wentworth DE, Drummond AJ, Smith GJ, Webster RG (2013) Influenza A virus migration and persistence in North American wild birds. PLoS Pathog 9(8):e1003570. doi:10.1371/journal.ppat.1003570
Barber MRW, Aldridge JR, Webster RG, Magor KE (2008) Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci USA 107:5913–5918
Becker WB (1966) The isolation and classification of Tern virus: influenza virus A/Tern/South Africa/1961. J Hyg 64:309–320
Bengtsson D, Safi K, Avril A, Fiedler W, Wikelski M, Gunnarsson G, Elmberg J, Tolf C, Olsen B, Waldenström J (2016) Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host? R Soc Open Sci 3(2):150633. doi:10.1098/rsos.150633
Bennett RS, Nezworski J, Velayudhan BT, Nagaraja KV, Zeman DH, Dyer N, Graham T, Lauer DC, Njenga MK, Halvorson DA (2004) Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America. Avian Dis 48(4):902–908. doi:10.1637/7208-051804r
Bentz P-G (1985) Studies on some urban mallard Anas platyrhynchos populations in Scandinavia. Part I: Causes of death, mortality and longevity among Malmö mallards as shown by ringing recoveries. Fauna Norv Ser C Cinclus 8:44–56
Berg M, Johansson M, Montell H, Berg AL (2001) Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol Infect 127(1):173–178
Berglund PG (2014) Exploring the epidemiology and population structure of Campylobacter jejuni in humans, broilers and wild birds. PhD Thesis, Linnaeus University, Kalmar, Sweden
Birdlife-International (2004) Birds in Europe: population estimates, trends and conservation status. Birdlife International, Cambridge, UK
Blanchong JA, Samuel MD, Mack G (2006) Multi-species patterns of avian cholera mortality in Nebraska’s Rainwater Basin. J wildl Dis 42(1):81–91
Bodewes R, Bestebroer TM, van der Vries E, Verhagen JH, Herfst S, Koopmans MP, Fouchier RA, Pfankuche VM, Wohlsein P, Siebert U, Baumgartner W, Osterhaus AD (2015) Avian influenza A(H10N7) virus-associated mass deaths among harbor seals. Emerg Infect Dis 21(4):720–722. doi:10.3201/eid2104.141675
Bonnedahl J, Hernandez J, Stedt J, Waldenstrom J, Olsen B, Drobni M (2014) Extended-spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae in Gulls, Alaska, USA. Emerg Infect Dis 20(5):897–899. doi:10.3201/eid2005.130325
Bordes F, Morand S (2011) The impact of multiple infections on wild animal hosts: a review. Infect Ecol Epidemiol 1. doi:10.3402/iee.v1i0.7346
Both GW, Sleigh MJ, Cox NJ, Kendal AP (1983) Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J Virol 48:52–60
Botzler RG (1991) Epizootiology of avian cholera in wildfowl. J wildl Dis 27(3):367–395
Botzler RG (2002) Avian cholera on north coast California: distinctive epizootiological features. Ann N Y Acad Sci 969:224–228
Bröjer C, Ågren EO, Uhlhorn H, Bernodt K, Mörner T, Jansson DS, Mattsson R, Zohari S, Thoren P, Berg M, Gavier-Widen D (2009) Pathology of natural highly pathogenic avian influenza H5N1 infection in wild tufted ducks (Aythya fuligula). J Vet Diagn Invest 21:579–587
Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE (2006) Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis 12:1663–1670
Brown JD, Stallknecht DE, Swayne DE (2008) Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis 14(1):136–142. doi:10.3201/eid1401.070740
Brown J, Poulson R, Carter D, Lebarbenchon C, Pantin-Jackwood M, Spackman E, Shepherd E, Killian M, Stallknecht D (2012) Susceptibility of avian species to North American H13 low pathogenic avian influenza viruses. Avian Dis 56(4 Suppl):969–975. doi:10.1637/10158-040912-Reg.1
Cavanagh D (2005) Coronaviruses in poultry and other birds. Avian Pathol 34(6):439–448. doi:10.1080/03079450500367682
Champagnon J (2011) Consequences of the introduction of individuals within harvested populations: the case of the mallard Anas platyrhynchos. PhD Thesis, University of Monpellier, Montpellier, France
Chen R, Holmes EC (2006) Avian influenza virus exhibits rapid evolutionary dynamics. Mol Biol Evol 23:2336–2341
Chen R, Holmes EC (2009) Frequent inter-species transmission and geographic subdivision in avian influenza viruses from wild birds. Virology 383:156–161
Chen R, Holmes EC (2010) Hitchhiking and the population genetic structure of avian influenza virus. J Mol Evol 70:98–105
Chen H, Smith GJD, Zhang SY, Qin K, Wang J, Li KS, Webster RG, Peiris JSM, Guan Y (2005) H5N1 virus outbreak in migratory waterfowl. Nature 436:191–192
Christensen JP, Dietz HH, Bisgaard M (1998) Phenotypic and genotypic characters of isolates of Pasteurella multocida obtained from back-yard poultry and from two outbreaks of avian cholera in avifauna in Denmark. Avian Pathol 27(4):373–381. doi:10.1080/03079459808419354
Chu DK, Leung CY, Gilbert M, Joyner PH, Ng EM, Tse TM, Guan Y, Peiris JS, Poon LL (2011) Avian coronavirus in wild aquatic birds. J Virol 85(23):12815–12820. doi:10.1128/JVI.05838-11
Chu DK, Leung CY, Perera HK, Ng EM, Gilbert M, Joyner PH, Grioni A, Ades G, Guan Y, Peiris JS, Poon LL (2012) A novel group of avian astroviruses in wild aquatic birds. J Virol 86(24):13772–13778. doi:10.1128/JVI.02105-12
Church DL (2004) Major factors affecting the emergence and re-emergence of infectious diseases. Clin Lab Med 24(3):559–586. doi:10.1016/j.cll.2004.05.008
Converse KA, Kidd GA (2001) Duck plague epizootics in the United States, 1967–1995. J Wildl Dis 37:347–357
Costa TP, Brown JD, Howerth EW, Stallknecht DE (2010) Effect of a prior exposure to a low pathogenic avian influenza virus in the outcome of a heterosubtypic low pathogenic avian influenza infection in mallards (Anas platyrhynchos). Avian Dis 54:1286–1291
Costa TP, Brown JD, Howerth EW, Stallknecht DE, Swayne DE (2011) Homo- and heterosubtypic low pathogenic avian influenza exposure on H5N1 highly pathogenic avian influenza virus infection in wood ducks (Aix sponsa). PLoS One 6(1):e15987. doi:10.1371/journal.pone.0015987
Cramp S, Simmons KEL (1977) Mallard (Anas platyrhynchos). In: Cramp S, Simmons KEL, Ferguson-Lees IJ et al (eds) Birds of the Western Palearctic, vol 1. Oxford University Press, London, pp 505–519
Cramp S, Brooks DJ, Dunn E, Gillmor R, Hollom PAD, Hudson R, Nicholson EM, Ogilvie MA, Olney PJS, Roselaar CS, Simmons KEL, Voos KH, Wallace DIM, Wattel J, Wilson MG (1985) Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic, vol 4. Oxford University Press, Oxford
Daly JM, MacRae S, Newton JR, Wattrang E, Elton DM (2011) Equine influenza: a review of an unpredictable virus. Vet J 189(1):7–14. doi:10.1016/j.tvjl.2010.06.026
Daoust P-Y, Kibenge FSB, Fouchier RAM, van de Bildt MWG, Kuiken T (2011) Replication of low pathogenic avian influenza virus in naturally infected mallard ducks (Anas platyrhynchos) causes no morphologic lesions. J Wildl Dis 47:401–409
De Benedictis P, Schultz-Cherry S, Burnham A, Cattoli G (2011) Astrovirus infections in humans and animals: molecular biology, genetic diversity, and interspecies transmissions. Infect Genet Evol 11(7):1529–1544. doi:10.1016/j.meegid.2011.07.24
de Graaf M, Osterhaus AD, Fouchier RA, Holmes EC (2008) Evolutionary dynamics of human and avian metapneumoviruses. J Gen Virol 89(Pt 12):2933–2942. doi:10.1099/vir.0.2008/006957-0
Delany S, Scott D (2006) Waterbird population estimates, 4th edn. Wetlands International, Netherlands
Delnatte P, Ojkic D, Delay J, Campbell D, Crawshaw G, Smith DA (2013) Pathology and diagnosis of avian bornavirus infection in wild Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) in Canada: a retrospective study. Avian Pathol 42(2):114–128. doi:10.1080/03079457.2013.769669
Delnatte P, Nagy E, Ojkic D, Leishman D, Crawshaw G, Elias K, Smith DA (2014) Avian bornavirus in free-ranging waterfowl: prevalence of antibodies and cloacal shedding of viral RNA. J Wildl Dis 50(3):512–523. doi:10.7589/2013-08-218
Descamps S, Jenouvrier S, Gilchrist HG, Forbes MR (2012) Avian cholera, a threat to the viability of an Arctic seabird colony? PloS one 7(2):e29659. doi:10.1371/journal.pone.0029659
Drilling N, Titman R, Mckinney F (2002) Mallard (Anas platyrhynchos). In: Poole A (ed) Birds of North America Online. Cornell Lab of Ornithology, Ithaca, doi: 10.2173/bna.658. http://bna.birds.cornell.edu/bna/species/658
Dugan VG, Chen R, Spiro DJ, Sengamalay N, Zaborsky J, Ghedin E, Nolting J, Swayne DE, Runstadler JA, Happ GM, Senne DA, Wang R, Slemons RD, Holmes EC, Taubenberger JK (2008) The evolutionary genetics and emergence of avian influenza A viruses in wild birds. PLoS Pathog 4:e1000076. doi:10.1371/journal/ppat/1000076
Dusek RJ, Hallgrimsson GT, Ip HS, Jonsson JE, Sreevatsan S, Nashold SW, TeSlaa JL, Enomoto S, Halpin RA, Lin X, Fedorova N, Stockwell TB, Dugan VG, Wentworth DE, Hall JS (2014) North Atlantic migratory bird flyways provide routes for intercontinental movement of avian influenza viruses. PLoS One 9(3):e92075. doi:10.1371/journal.pone.0092075
Ellis TM, Bousfield RB, Bissett LA, Dyrting KC, Luk GS, Tsim ST, Sturm-Ramirez K, Webster RG, Guan Y, Malik Peiris JS (2004) Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol 33(5):492–505. doi:10.1080/03079450400003601
Engering A, Hogerwerf L, Slingenbergh J (2013) Pathogen-host-environment interplay and disease emergence. Emerg Microbes Infect 2(2):e5. doi:10.1038/emi.2013.5
European Food Safety Authority (2014) Highly pathogenic avian influenza A subtype H5N8. EFSA J 12:3941
FAO (2012) H5N1 Highly pathogenic avian influenza global review. EMPRES/GLEW Report, FAO Issue 31. http://www.fao.org/docrep/015/an388e/an388e.pdf
Fawaz M, Vijayakumar P, Mishra A, Gandhale PN, Dutta R, Kamble NM, Sudhakar SB, Roychoudhary P, Kumar H, Kulkarni DD, Raut AA (2016) Duck gut viral metagenome analysis captures snapshot of viral diversity. Gut Pathog 8:30. doi:10.1186/s13099-016-0113-5
Feare CJ (2010) Role of wild birds in the spread of highly pathogenic avian influenza virus H5N1 and implications for global surveillance. Avian Dis 54:201–212
Fenton A, Pedersen AB (2005) Community epidemiology framework for classifying disease threats. Emerg Infect Dis 11(12):1815–1821. doi:10.3201/eid1112.050306
Fereidouni SR, Starick E, Beer M, Wilking H, Kalthoff D, Grund C, Häuslaigner R, Breithaupt A, Lange E, Harder TC (2009) Highly pathogenic avian influenza virus infection of mallards with homo- and heterosubtypic immunity induced by low pathogenic avian influenza viruses. PLoS One 4:e6706. doi:10.1371/journal.pone.0006706
Fereidouni SR, Grund C, Häuslaigner R, Lange E, Wilking H, Harder TC, Beer M, Starick E (2010) Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenic avian influenza viruses. Avian Dis 54:79–85
Fereidouni SR, Harder TC, Globig A, Starick E (2014) Failure of productive infection of mallards (Anas platyrhynchos) with H16 subtype of avian influenza viruses. Influenza Other Respir Virus 8(6):613–616. doi:10.1111/irv.12275
Flint PL, Franson JC (2009) Does influenza A affect body condition of wild mallard ducks, or vice versa? Proc R Soc B 276:2345–2346
Fu Y, Pan M, Wang X, Xu Y, Xie X, Knowles NJ, Yang H, Zhang D (2009) Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J Gen Virol 90(Pt 5):1104–1108. doi:10.1099/vir.0.008599-0
Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y (2013) Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368(20):1888–1897. doi:10.1056/NEJMoa1304459
Gauthier-Clerc M, Lebarbenchon C, Thomas F (2007) Recent expansion of highly pathogenic avian influenza H5N1: a critical review. Ibis 149:202–214
George TL, Harrigan RJ, LaManna JA, DeSante DF, Saracco JF, Smith TB (2015) Persistent impacts of West Nile virus on North American bird populations. Proc Natl Acad Sci U S A 112(46):14290–14294. doi:10.1073/pnas.1507747112
Gething MJ, Bye J, Skehel JJ, Wakefield M (1980) Cloning and DNA sequence of double-stranded copies of hemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature 287:310–306
Gonzalez-Reiche AS, Morales-Betoulle ME, Alvarez D, Betoulle JL, Muller ML, Sosa SM, Perez DR (2012) Influenza A viruses from wild birds in Guatemala belong to the North American lineage. Plos One 7(3):e32873. doi:10.1371/journal.pone.0032873
Gordus AG (1993) Notes on the first known avian cholera epizootic in wildfowl in North America. J Wildl Dis 29(2):367
Grard G, Moureau G, Charrel RN, Lemasson JJ, Gonzalez JP, Gallian P, Gritsun TS, Holmes EC, Gould EA, de Lamballerie X (2007) Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 361(1):80–92. doi:10.1016/j.virol.2006.09.015
Groth M, Lange J, Kanrai P, Pleschka S, Scholtissek C, Krumbholz A, Platzer M, Sauerbrei A, Zell R (2014) The genome of an influenza virus from a pilot whale: relation to influenza viruses of gulls and marine mammals. Infect Genet Evol 24C:183–186. doi:10.1016/j.meegid.2014.03.026
Guo J, Shivaprasad HL, Rech RR, Heatley JJ, Tizard I, Payne S (2014) Characterization of a new genotype of avian bornavirus from wild ducks. Virology J 11(1):197. doi:10.1186/s12985-014-0197-9
Guy JS (2000) Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses: a review. Avian Pathol 29(3):207–212. doi:10.1080/03079450050045459
Hald B, Skov MN, Nielsen EM, Rahbek C, Madsen JJ, Waino M, Chriel M, Nordentoft S, Baggesen DL, Madsen M (2016) Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet Scand 58:11. doi:10.1186/s13028-016-0192-9
Hasan B, Melhus A, Sandegren L, Alam M, Olsen B (2014) The gull (Chroicocephalus brunnicephalus) as an environmental bioindicator and reservoir for antibiotic resistance on the coastlines of the Bay of Bengal. Microb Drug Resist 20(5):466–471. doi:10.1089/mdr.2013.0233
Hatchette TF, Walker D, Johnson C, Baker A, Pryor SP, Webster RG (2004) Influenza A viruses in feral Canadian ducks: extensive reassortment in nature. J Gen Virol 85:2327–2337
Hernandez J, Johansson A, Stedt J, Bengtsson S, Porczak A, Granholm S, Gonzalez-Acuna D, Olsen B, Bonnedahl J, Drobni M (2013) Characterization and comparison of extended-spectrum beta-lactamase (ESBL) resistance genotypes and population structure of Escherichia coli isolated from Franklin’s gulls (Leucophaeus pipixcan) and humans in Chile. PLoS One 8(9):e76150. doi:10.1371/journal.pone.0076150
Hill NJ, Takekawa JY, Ackerman JT, Hobson KA, Herring G, Cardona CJ, Runstadler JA, Boyce WM (2012) Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos). Mol Ecol 21(24):5986–5999. doi:10.1111/j.1365-294X.2012.05735.x
Hill SC, Manvell RJ, Schulenburg B, Shell W, Wikramaratna PS, Perrins CM, Sheldon BC, Brown IH, Pybus OG (2016) Antibody responses to avian influenza viruses in wild birds broadens with age. Proc R Soc B 283. doi:10.1098/rspb.2016.2159
Hoque MA, Burgess GW, Karo-Karo D, Cheam AL, Skerratt LF (2012) Monitoring of wild birds for Newcastle disease virus in north Queensland, Australia. Prev Vet Med 103(1):49–62. doi:10.1016/j.prevetmed.2011.08.013
Hoye B (2011) Host–pathogen interactions on the move: migratory waterfowl and avian influenza viruses. Utrecht University, The Netherlands
Hoye BJ, Munster VJ, Nishiura H, Klaassen M, Fouchier RAM (2010) Surveillance of wild birds for avian influenza virus. Emerg Infect Dis 16(12):1827–1834. doi:10.3201/Eid1612.100589
Hoye B, Munster VJ, Huig N, de Vries P, Oosterbeek K, Tijsen W, Klaassen M, Fouchier RAM, van Gils JA (2016) Hampered performance of migratory swans: intra- and inter-seasonal effects of avian influenza virus. Integr Comp Biol 56:317–329
Huang Y, Robertson GJ, Ojkic D, Whitney H, Lang AS (2014a) Diverse inter-continental and host lineage reassortant avian influenza A viruses in pelagic seabirds. Infect Genet Evol 22:103–111. doi:10.1016/j.meegid.2014.01.014
Huang Y, Wille M, Benkaroun J, Munro H, Bond AL, Fifield DA, Robertson GJ, Ojkic D, Whitney H, Lang AS (2014b) Perpetuation and reassortment of gull influenza A viruses in Atlantic North America. Virology 456-457:353–363. doi:10.1016/j.virol.2014.04.009
Hughes LA, Savage C, Naylor C, Bennett M, Chantrey J, Jones R (2009) Genetically diverse coronaviruses in wild bird populations of northern England. Emerg Infect Dis 15(7):1091–1094. doi:10.3201/eid1507.090067
Ip HS, Flint PL, Franson JC, Dusek RJ, Derkson DV, Gill RE Jr, Ely CE, Pearce JM, Lanctot RB, Matsuoka SM, Irons DB, Fischer JB, Oates RM, Petersen MR, Fondell TF, Rocque DA, Pedersen JC, Rothe TC (2008) Prevalence of influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virol J 5:71–81
Ito T, Okazaki K, Kawaoka Y, Takada A, Webster RG, Kida H (1995) Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch Virol 140:1163–1172
Jackwood MW, Hall D, Handel A (2012) Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol 12(6):1305–1311. doi:10.1016/j.meegid.2012.05.003
Jeon WJ, Lee EK, Joh SJ, Kwon JH, Yang CB, Yoon YS, Choi KS (2008) Very virulent infectious bursal disease virus isolated from wild birds in Korea: epidemiological implications. Virus Res 137(1):153–156. doi:10.1016/j.virusres.2008.06.013
Jindal N, Chander Y, Chockalingam AK, de Abin M, Redig PT, Goyal SM (2009) Phylogenetic analysis of Newcastle disease viruses isolated from waterfowl in the upper midwest region of the United States. Virol J 6:191. doi:10.1186/1743-422X-6-191
Jonassen CM, Kofstad T, Larsen IL, Lovland A, Handeland K, Follestad A, Lillehaug A (2005) Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J Gen Virol 86(6):1597–1607. doi:10.1099/vir.0.80927-0
Jones R, Guneratne J (1984) The pathogenicity of some avian reoviruses with particular reference to tenosynovitis. Avian Pathol 13:173–189
Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Bröjer C, Sahlin S, Svensson L, Waldenström J, Lundkvist Å, Olsen B (2010) Influenza virus in a natural host, the mallard: experimental infection data. PLoS One 5:e8935. doi:10.1371/journal.pone.0008935
Kageyama T, Fujisaki S, Takashita E, Xu H, Yamada S, Uchida Y, Neumann G, Saito T, Kawaoka Y, Tashiro M (2013) Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance 18(15):20453
Kapczynski DR, Swayne DE (2009) Influenza vaccines for avian species. Curr Top Microbiol Immunol 333:133–152. doi:10.1007/978-3-540-92165-3_6
Kawaoka Y, Cox NJ, Haller O, Hongo S, Klenk H-D, Lamb RA, McCauley J, Palese P, Rimstad E, Webster RG (2005) Orthomyxoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy: eighth report of the international committee for the taxonomy of viruses. Elsevier Academic Press, San Diego, USA, pp 681–693
Keawcharoen J, van Riel G, van Amerongen G, Bestebroer TM, Beyer WEP, van Lavieren R, Osterhaus ADME, Fouchier RAM, Kuiken T (2008) Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 14:600–606
Kibenge FS, Dhillon AS, Russell RG (1988) Biochemistry and immunology of infectious bursal disease virus. J Gen Virol 69:1757–1775
Kim BY, Lee DH, Kim MS, Jang JH, Lee YN, Park JK, Yuk SS, Lee JB, Park SY, Choi IS, Song CS (2012) Exchange of Newcastle disease viruses in Korea: the relatedness of isolates between wild birds, live bird markets, poultry farms and neighboring countries. Infect Genet Evol 12(2):478–482. doi:10.1016/j.meegid.2011.12.004
Kleijn D, Munster VJ, Ebbinge BS, Jonkers DA, Müskens GJDM, van Randen Y, Fouchier RAM (2010) Dynamics and ecological consequences of avian influenza virus infection in greater white-fronted geese in thier winter staging areas. Proc R Soc B 277:2041–2048
Koci D, Schultz-Cherry S (2002) Avian astroviruses. Avian Pathol 31(3):213–227. doi:10.1080/03079450220136521
Koehler AV, Pearce JM, Flint PL, Franson JC, Ip HS (2008) Genetic evidence of interncontinental movement of avian influenza in a migratory bird: the Northern Pintail (Anas acuta). Mol Ecol 17:4754–4762
Koel BF, Burke DF, Bestebroer TM, van der Vliet S, Zondag GC, Vervaet G, Skepner E, Lewis NS, Spronken MI, Russell CA, Eropkin MY, Hurt AC, Barr IG, de Jong JC, Rimmelzwaan GF, Osterhaus AD, Fouchier RA, Smith DJ (2013) Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 342(6161):976–979. doi:10.1126/science.1244730
Kraus RH, Zeddeman A, van Hooft P, Sartakov D, Soloviev SA, Ydenberg RC, Prins HH (2011) Evolution and connectivity in the world-wide migration system of the mallard: inferences from mitochondrial DNA. BMC Genet 12:99. doi:10.1186/1471-2156-12-99
Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG (2010) Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological ‘hot spot’ for influenza viruses. Proc Biol Sci 277(1699):3373–3379. doi:10.1098/rspb.2010.1090
Krauss S, Stallknecht DE, Slemons RD, Bowman AS, Poulson RL, Nolting JM, Knowles JP, Webster RG (2016) The engima of the apparent dissapearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proc Natl Acad Sci USA 113(32):9033–9038
Kuiken T (2013) Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc Biol Sci 280(1763):20130990. doi:10.1098/rspb.2013.0990
Kuiken T, Frandsen D, Clavijo A (1998) Newcastle disease in cormorants. Can Vet J La revue veterinaire canadienne 39(5):299
LaDeau SL, Kilpatrick AM, Marra PP (2007) West Nile virus emergence and large-scale declines of North American bird populations. Nature 447(7145):710–713. doi:10.1038/nature05829
Lambert V, Cova L, Chevallier P, Mehrotra R, Trepo C (1991) Natural and experimental infection of wild mallard ducks with duck hepatitis B virus. J Gen Virol 72:417–420
Lang AS, Lebarbenchon C, Ramey AM, Robertson GJ, Waldenström J, Wille M (2016) Assessing the role of seabirds in the ecology of influenza A viruses. Avian Dis 60:378–386
Latorre-Margalef N, Gunnarsson G, Munster VJ, Fouchier RAM, Osterhaus ADME, Elmberg J, Olsen B, Wallensten A, Fransson T, Brudin L, Waldenström J (2009a) Does influenza A affect body condition of wild mallard ducks, or vice versa? A reply to flint and franson. Proc R Soc B 276:2347–2349
Latorre-Margalef N, Gunnarsson G, Munster VJ, Fouchier RAM, Osterhaus ADME, Elmberg J, Olsen B, Wallensten A, Haemig PD, Fransson T, Brudin L, Waldenström J (2009b) Effects of influenza A virus infection on migrating mallard ducks. Proc R Soc B 276:1029–1036
Latorre-Margalef N (2012) Ecology and evolution of influenza A virus in Mallards Anas platyrhynchos. Doctoral thesis, Linnaeus University Press. ISBN: 978-91-86983-61-1
Latorre-Margalef N, Grosbois V, Wahlgren J, Munster VJ, Tolf C, Fouchier RAM, Osterhaus ADME, Waldenström J, Olsen B (2013) Heterosubtypic immunity to influenza A virus infections in mallards may explain existence of multiple virus subtypes. PLoS Pathog 9(6):e1003443. doi:10.1371/journal.ppat.1003443
Latorre-Margalef N, Tolf C, Grosbois V, Avril A, Bengtsson D, Wille M, Osterhaus ADME, Fouchier RAM, Olsen B, Waldenström J (2014) Long-term variation in influenza A virus prevalence and subtype diversity in a migratory mallards in Northern Europe. Proc R Soc B 281:20140098. doi:10.1098/rspb.2014.0098
Latorre-Margalef N, Avril A, Tolf C, Olsen B, Waldenstrom J (2016a) How does sampling methodology influenza molecular detection and isolation success in influenza virus field samples? Appl Environ Microbiol 82:1147–1153
Latorre-Margalef N, Brown JD, Fojtik A, Poulson RL, Carter D, Franca M, Stallknecht DE (2016b) Competition between IAV subtypes through heterosubtypic immunity modulates re-infection and antibody dynamics in the mallard reservoir. bioRxiv Preprint
Lebarbenchon C, Sreevatsan S, Lefèvre T, Yang M, Ramakrishnan MA, Brown JD, Stallknecht DE (2012) Reassortment influenza A viruses in wild duck populations: effects on viral shedding and persistence in water. Proc R Soc B. doi:10.1098/rspb.2012.1271
Lee YJ, Kang HM, Lee EK, Song BM, Jeong J, Kwon YK, Kim HR, Lee KJ, Hong MS, Jang I, Choi KS, Kim JY, Lee HJ, Kang MS, Jeong OM, Baek JH, Joo YS, Park YH, Lee HS (2014) Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis 20(6):1087–1089. doi:10.3201/eid2006.140233
Lee DH, Torchetti MK, Winker K, Ip HS, Song CS, Swayne DE (2015) Intercontinental spread of Asian-origin H5N8 to North America through Beringia by migratory birds. J Virol 89(12):6521–6524. doi:10.1128/Jvi.00728-15
Leighton FA, Heckert RA (2007) Newcastle disease and related avian paramyxoviruses. In: Thomas NJ, Hunter BD, Atkinson CT (eds) Infectious diseases of wild birds. Blackwell, Ames, pp 3–16
Lindh E, Huovilainen A, Ratti O, Ek-Kommonen C, Sironen T, Huhtamo E, Poysa H, Vaheri A, Vapalahti O (2008) Orthomyxo-, paramyxo- and flavivirus infections in wild waterfowl in Finland. Virol J 5:35. doi:10.1186/1743-422X-5-35
Lindstrom SE, Cox NJ, Klimov AI (2004) Genetic analysis of human H2N2 and early H3N2 influenza viruses, 1957–1972: evidence for genetic divergence and multiple reassortment events. Virology 328:101–119
Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, Zhang XL, Zhao D, Wang G, Feng Y, Ma J, Liu W, Wang J, Gao GF (2005) Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 309(5738):1206. doi:10.1126/science.1115273
Lobo FP, Mota BE, Pena SD, Azevedo V, Macedo AM, Tauch A, Machado CR, Franco GR (2009) Virus-host coevolution: common patterns of nucleotide motif usage in Flaviviridae and their hosts. PLoS One 4(7):e6282. doi:10.1371/journal.pone.0006282
Lundqvist ML, Middleton DL, Radford C, Warr GW, Magor KE (2006) Immunoglobulins of the non-galliform birds: antibody expression and repertoire in the duck. Dev Comp Immunol 30(1–2):93–100. doi:10.1016/j.dci.2005.06.019
Ma EJ, Hill NJ, Zabilansky J, Yuan K, Runstadler JA (2016) Reticulate evolution is favored in influenza niche switching. Proc Natl Acad Sci USA 113(19):5335–5339. doi:10.1073/pnas.1522921113
Magor KE (2011) Immunoglobulin genetics and antibody responses to influenza in ducks. Dev Comp Immunol 35:1008–1016
Magor KE, Warr GW, Bando Y, Middleton DL, Higgins DA (1998) Secretory immune system of the duck (Anas platyrhynchos). Identification and expression of the genes encoding IgA and IgM heavy chains. Eur J Immunol 28:1063–1068
Magor KE, Higgins DA, Middleton DL, Warr GW (1999) Opposite orientation of the alpha- and upsilon-chain constant region genes in the immunoglobulin heavy chain locus of the duck. Immunogenetics 49:692–695
Maxted AM, Luttrell MP, Goekjian VH, Brown JD, Niles LJ, Dey AD, Kalasz KS, Swayne DE, Stallknecht DE (2012) Avian influenza virus infection dynamics in shorebird hosts. J Wildl Dis 48(2):322–334. doi:10.7589/0090-3558-48.2.322
McClintock BT, Nichols JD, Bailey LL, MacKenzie DI, Kendall WL, Franklin AB (2010) Seeking a second opinion: uncertainty in disease ecology. Ecol Lett 13:659–674
McDonald SM, Matthijnssens J, McAllen JK, Hine E, Overton L, Wang S, Lemey P, Zeller M, Van Ranst M, Sprio DJ, Patton JT (2009) Evolutionary dynamics of human rotaviruses: balancing reassortment with preferred genome constellations. PLoS Pathog 5:e1000634. doi:10.1371/journal.ppat.1000634
Morse SS (1995) Factors in the emergence of infectious diseases. Emerg Infect Dis 1(1):7–15. doi:10.3201/eid0101.950102
Morse SS (2004) Factors and determinants of disease emergence. Rev Sci Tech OIE 23:443–451
Munster V, Fouchier RAM (2009) Avian influenza virus: of virus and bird ecology. Vaccine 27:6340–6344
Munster VJ, Baas C, Lexmond P, Waldenström J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WEP, Schutten M, Olsen B, Osterhaus ADME, Fouchier RAM (2007) Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog 3:e61. doi:10.1371/journal.ppat.0030061
Muradrasoli S, Mohamed N, Hornyak A, Fohlman J, Olsen B, Belak S, Blomberg J (2009) Broadly targeted multiprobe QPCR for detection of coronaviruses: coronavirus is common among mallard ducks (Anas platyrhynchos). J Virol Methods 159(2):277–287. doi:10.1016/j.jviromet.2009.04.022
Muradrasoli S, Balint A, Wahlgren J, Waldenstrom J, Belak S, Blomberg J, Olsen B (2010) Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS One 5(10):e13640. doi:10.1371/journal.pone.0013640
Mushtaq MH, Juan H, Jiang P, Li Y, Li T, Du Y, Mukhtar MM (2008) Complete genome analysis of a highly pathogenic H5N1 influenza A virus isolated from a tiger in China. Arch Virol 153(8):1569–1574. doi:10.1007/s00705-008-0145-3
Nayak B, Kumar S, DiNapoli JM, Paldurai A, Perez DR, Collins PL, Samal SK (2010) Contributions of the avian influenza virus HA, NA, and M2 surface proteins to the induction of neutralizing antibodies and protective immunity. J Virol 84(5):2408–2420. doi:10.1128/JVI.02135-09
Nelson MI, Pollett S, Ghersi B, Silva M, Simons MP, Icochea E, Gonzalez AE, Segovia K, Kasper MR, Montgomery JM, Bausch DG (2016) The genetic diversity of influenza A viruses in wild birds in Peru. PLoS One 11(1):e0146059. doi:10.1371/journal.pone.0146059
Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM (2006) Global patterns of influenza A virus in wild birds. Science 312:384–388
Olson SH, Parmley J, Soos C, Gilbert M, Latorre-Margalef N, Hall JS, Hansbro PM, Leighton F, Munster V, Joly D (2014) Sampling strategies and biodiversity of influenza A subtypes in wild birds. PLoS One 9(3):e90826. doi:10.1371/journal.pone.0090826
Pantin-Jackwood MJ, Swayne DE (2007) Pathobiology of asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Dis 50:250–259
Pantin-Jackwood MJ, Strother KO, Mundt E, Zsak L, Day JM, Spackman E (2011) Molecular characterization of avian astroviruses. Arch Virol 156(2):235–244. doi:10.1007/s00705-010-0849-z
Pasick J, Berhane Y, Embury-Hyatt C, Copps J, Kehler H, Handel K, Babiuk S, Hooper-McGrevy K, Li Y, Mai Le Q, Lien Phuong S (2007) Susceptibility of Canada Geese (Branta canadensis) to highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 13(12):1821–1827. doi:10.3201/eid1312.070502
Pasick J, Berhane Y, Joseph T, Bowes V, Hisanaga T, Handel K, Alexandersen S (2015) Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci Rep 5:9484. doi:10.1038/srep09484
Pearce JM, Ramey AM, Ip HS, Gill REJ (2009) Limited evidence of trans-hemispheric movement of avian influenza viruses among contemporary North American shorebird isolates. Virus Res 148:44–50
Pearce JM, Reeves AB, Ramey AM, Hupp JW, Ip HS, Bertram M, Petrula MJ, Scotton BD, Trust KA, Meixell BW, Runstadler JA (2011) Interspecific exchange of avian influenza virus genes in Alaska: the influenza of trans-hemispheric migratory tendency and breeding ground sympatry. Mol Ecol 20:1015–1025
Pereda AJ, Uhart M, Perez AA, Zaccagnini ME, La Sala L, Decarre J, Goijman A, Solari L, Suarez R, Craig MI, Vagnozzi A, Rimondi A, Konig G, Terrera MV, Kaloghlian A, Song H, Sorrell EM, Perez DR (2008) Avian influenza virus isolated in wild waterfowl in Argentina: evience of a potentially unqie phylogenetic lineage in South America. Virology 378:363–370
Perkins LE, Swayne DE (2001) Pathobiology of A/chicken/Hong Kong/220/97(H5N1) avian influenza virus in seven gallinaceous species. Vet Pathol 38:149–164
Perkins LEL, Swayne DE (2002) Susceptibility of Laughing Gulls (Larus atricilla) to H5N1 and H5N2 highly pathogenic avian influenza viruses. Avian Dis 46:877–885
Pybus OG, Rambaut A (2009) Evolutionary analysis of the dynamics of viral infectious disease. Nat Rev Genet 10(8):540–550. doi:10.1038/nrg2583
Rabadan R, Levine AJ, Robins H (2006) Comparison of avian and human influenza A viruses reveals a mutational bias on the viral genomes. J Virol 80:11887–11891
Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC (2008a) The genomic and epidemiological dynamics of human influenza A virus. Nature 453:615–619
Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC (2008b) The genomic and epidemiological dynamics of human influenza A virus. Nature 453(7195):615–619. doi:10.1038/nature06945
Ramey AM, Pearce JM, Flint PL, Ip HS, Derkson DV, Franson JC, Petrula MJ, Scotton BD, Sowl KM, Wege ML, Trust KA (2010) Intercontinental reassortment and genomic variation of low pathogenic avian influenza viruses isolated form Nothern Pintails (Anas acuta) in Alaska: examining the evidence through space and time. Virology 401:179–189
Ramey AM, Reeves AB, Ogawa H, Ip HS, Imai K, Bui VN, Yamaguchi E, Silko NY, Afonso CL (2013) Genetic diversity and mutation of avian paramyxovirus serotype 1 (Newcastle disease virus) in wild birds and evidence for intercontinental spread. Arch Virol 158(12):2495–2503. doi:10.1007/s00705-013-1761-0
Ramey AM, Poulson RL, Gonzalez-Reiche AS, Wilcox BR, Walther P, Link P, Carter DL, Newsome GM, Muller ML, Berghaus RD, Perez DR, Hall JS, Stallknecht DE (2014) Evidence for seasonal patterns in the relative abundance of avian influenza virus subtypes in blue-winged teal (Anas discors). J Wildl Dis 50(4):916–922. doi:10.7589/2013-09-232
Ramey AM, Pearce JM, Reeves AB, Poulson RL, Dobson J, Lefferts B, Spragens K, Stallknecht DE (2016) Surveillance for Eurasian-origin and intercontinental reassortant highly pathogenic influenza A viruses in Alaska, spring and summer 2015. Virol J 13(1):55. doi:10.1186/s12985-016-0511-9
Ramos R, Garnier R, Gonzalez-Solis J, Boulinier T (2014) Long antibody persistence and transgenerational transfer of immunity in a long-lived vertebrate. Am Nat 184(6):764–776. doi:10.1086/678400
Ren H, Jin Y, Hu M, Zhou J, Song T, Huang Z, Li B, Li K, Zhou W, Dai H, Shi W, Yue J, Liang L (2016) Ecological dynamics of influenza A viruses: cross-species transmission and global migration. Sci Rep 6:36839. doi:10.1038/srep36839
Roche B, Lebarbenchon C, Gauthier-Clerc M, Chang CM, Thomas F, Renaud F, van der Werf S, Guegan JF (2009) Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005–2006 epidemics in the Camargue area. Infect Genet Evol 9(5):800–805. doi:10.1016/j.meegid.2009.04.009
Roche B, Drake JM, Brown J, Stallknecht DE, Bedford T, Rohani P (2014) Adaptive evolution and environmental durability jointly structure phylodynamic patterns in avian influenza viruses. PLoS Biol 12(8):e1001931. doi:10.1371/journal.pbio.1001931
Roger F, Caron A, Morand S, Pedrono M, de Garine-Wichatitsky M, Chevalier V, Tran A, Gaidet N, Figuie M, de Visscher MN, Binot A (2016) One Health and EcoHealth: the same wine in different bottles? Infect Ecol Epidemiol 6:30978. doi:10.3402/iee.v6.30978
Rosseel T, Lambrecht B, Vandenbussche F, van den Berg T, Van Borm S (2011) Identification and complete genome sequencing of paramyxoviruses in mallard ducks (Anas platyrhynchos) using random access amplification and next generation sequencing technologies. Virol J 8:463. doi:10.1186/1743-422X-8-463
Runstadler J, Happ G, Slemons RD, Sheng Z-M, Gundlach N, Petrula M, Senne D, Nolting J, Evers DL, Modrell A, Huson H, Hills S, Rothe T, Marr T, Taubenberger JK (2007) Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks at Minto Flats Refuge, Alaska, during August 2005. Arch Virol 152(10):1901–1910
Samji T (2009) Influenza A: understanding the viral life cycle. Yale J Biol Med 82:153–159
Samuel MD, Shadduck DJ, Goldberg DR (2005a) Avian cholera exposure and carriers in greater white-fronted geese breeding in Alaska, USA. J Wildl Dis 41(3):498–502
Samuel MD, Shadduck DJ, Goldberg DR, Johnson WP (2005b) Avian cholera in waterfowl: the role of lesser snow and ross’s geese as disease carriers in the Playa Lakes Region. J Wildl Dis 41(1):48–57
Samuel M, Botzler R, Wobeser G (2007) Avian cholera. In: Thomas N, Hunter B, Atkinson C (eds) Infectious diseases of wild birds. Blackwell, Iowa, USA, pp 239–269
Schmid Hempel P (2011) Evolutionary parasitology. The integrated study of infections, immunology, ecology, and genetics. Oxford University Press, Oxford, UK
Scholtissek C, Rohde W, Von Hoyningen C, Rott R (1978) On the origin of human influenza virus subtypes H2N2 and H3N2. Virology 87:13–20
Segovia KM, Stallknecht DE, Kapczynski DR, Stabler L, Berghaus RD, Fotjik A, Latorre-Margalef N, Franca MS (2017) Adaptive heterosubtypic immunity to low pathogenic avian influenza viruses in experimentally infected mallards. Plos One 12(1):e0170335. doi:10.1371/journal.pone.0170335
Sharp GB, Kawaoka Y, Jones DJ, Bean WJ, Pryor SP, Hinshaw V, Webster RG (1997) Coinfection of wild ducks by influenza A viruses: distribution patterns and biological significance. J Virol 71(8):6128–6135
Shin H-J, Njenga MK, McComb B, Halvorson DA, Nagaraja KV (2000) Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. J Clin Microbiol 38(11):4282–4284
Shin H-J, Nagaraja KV, McComb B, Halvorson DA, Jirjis FF, Shaw DP, Seal BS, Njenga MK (2002) Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys. Virus Res 83:207–212
Slemons RD, Easterday BC (1978) Virus replication in the digestive tract of ducks exposed by aerosol to type-A influenza. Avian Dis 22:367–377
Slusher MJ, Wilcox BR, Lutrell MP, Poulson RL, Brown JD, Yabsley MJ, Stallknecht DE (2014) Are passerine birds reservoirs for influenza a viruses? J Wildl Dis. doi:10.7589/2014-02-043
Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA (2004) Mapping the antigenic and genetic evolution of influenza virus. Science 305(5682):371–376. doi:10.1126/science.1097211
Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y (2006a) Avian influenza H5N1 in naturally infected domestic cat. Emerg Infect Dis 12(4):681–683. doi:10.3201/eid1204.051396
Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Chutinimitkul S, Thanawongnuwech R, Poovorawan Y (2006b) Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis 12(11):1744–1747. doi:10.3201/eid1211.060542
Stallknecht DE, Brown JD (2007) Wild birds and the epidemiology of avian influenza. J Wildl Dis 43:S15–S20
Stedt J, Bonnedahl J, Hernandez J, McMahon BJ, Hasan B, Olsen B, Drobni M, Waldenstrom J (2014) Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect Ecol Epidemiol 4. doi:10.3402/iee.v4.21565
Steel J, Lowen AC (2014) Influenza a virus reassortment. Curr Top Microbiol Immunol 385:377–401. doi:10.1007/82_2014_395
Strong T, Dowd S, Gutierrez AF, Molnar D, Coffman J (2013) Amplicon pyrosequencing and ion torrent sequencing of wild duck eubacterial microbiome from fecal samples reveals numerous species linked to human and animal diseases. F1000Research 2(224). doi:10.12688/f1000research.2-224.v2
Suarez DL (2000) Evolution of avian influenza viruses. Vet Microbiol 74(1-2):15–27
Swayne DE, Suarez DL (2000) Highly pathogenic avian influenza. Rev Sci Tech 19:463–482
Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG (2005) Characterization of the 1918 influenza virus polymerase genes. Nature 437:889–893
Thangavel RR, Reed A, Norcross EW, Dixon SN, Marquart ME, Stray SJ (2011) “Boom” and “Bust” cycles in virus growth suggest multiple selective forces in influenza a evolution. Virology J 8:180. doi:10.1186/1743-422X-8-180
Todd D, Smyth VJ, Ball NW, Donnelly BM, Wylie M, Knowles NJ, Adair BM (2009) Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol 38(1):21–30. doi:10.1080/03079450802632056
Tolf C, Latorre-Margalef N, Wille M, Bengtsson D, Gunnarsson G, Grosbois V, Hasselquist D, Olsen B, Elmberg J, Waldenström J (2013a) Individual variation in influenza A virus infection histories and long-term immune responses in mallards. PLoS One 8(4):e61201. doi:10.1371/journal.pone.0061201
Tolf C, Wille M, Haidar A-K, Avril A, Zohari S, Waldenström J (2013b) Prevalence of avian paramyxovirus type 1 in mallards during autumn migration in the western Baltic Sea region. Virol J 10:285. doi:10.1186/1743-422X-10-285
USFWS (2003) Review of captive-bred mallard regulatons on shooting preserves. Division of Migratory Bird Management, U. S. Fish and Wildlife Service, Washington, USA.
USFWS (2011) Waterfowl population status, 2011. U. S. Department of the Interior, USA
USGS (1999) Aspergillosis. In: Franson MFaJC (ed) Field manual of wildlife diseases: birds. USGS, pp 129–134
van Dijk JGB, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, Klaassen M (2014) Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J Anim Ecol 83(1):266–275. doi:10.1111/1365-2656.12131
van Dijk J, Kleyheeg E, Soons M, Nolet B, Fouchier R, Klaassen M (2015a) Weak negative associations between avian influenza virus infection and movement behaviour in a key host species, the mallard Anas platyrhynchos. Oikos 10:1293–1303. doi:10.1111/oik.01836
van Dijk JGB, Fouchier RAM, Klaassen M, Matson KD (2015b) Minor differences in body condition and immune status between avian influenza virus-infected and noninfected mallards: a sign of coevolution? Ecol Evol 5(2):436–449. doi:10.1002/Ece3.1359
van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RAM, Klaassen M (2007) Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS One 2(1):e184. doi:10.1371/journal.pone.0000184
Vanderven HA, Petkau K, Ryan-Jean KE, Aldridge JR Jr, Webster RG, Magor KE (2012) Avian influenza rapidly induces antiviral genes in duck lung and intestine. Mol Immunol 51(3-4):316–324. doi:10.1016/j.molimm.2012.03.034
Verhagen JH, Herfst S, Fouchier RAM (2015) How a virus travels the world. Science 347:616–617
Vincent A, Awada L, Brown I, Chen H, Claes F, Dauphin G, Donis R, Culhane M, Hamilton K, Lewis N, Mumford E, Nguyen T, Parchariyanon S, Pasick J, Pavade G, Pereda A, Peiris M, Saito T, Swenson S, Van Reeth K, Webby R, Wong F, Ciacci-Zanella J (2014) Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health 61(1):4–17. doi:10.1111/zph.12049
Wang G, Zhan D, Li L, Lei F, Liu B, Liu D, Xiao H, Feng Y, Li J, Yang B, Yin Z, Song X, Zhu X, Cong Y, Pu J, Wang J, Liu J, Gao GF, Zhu Q (2008) H5N1 avian influenza re-emergence of Lake Qinghai: phylogenetic and antigenic analyses of the newly isolated viruses and roles of migratory birds in virus circulation. J Gen Virol 89:697–702
Warr GW, Magor KE, Higgins DA (1995) IgY: clues to the origins of modern antibodies. Immunol Today 16:392–398
Webster RG, Kawaoka Y (1988) Avian influenza. CRC Crit Rev Poult Biol 1:211–246
Webster RG, Rott R (1987) Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell 50:665–666
Webster RG, Morita M, Pridgen C, Tumova B (1976) Ortho- and paramyxoviruses from migrating feral ducks: characterization of a new group of influenza A viruses. J Gen Virol 32:317–225
Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murt KC (1978) Intestinal influenza: replication and characterization of influena viruses in ducks. Virology 84(2):268–278
Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y (1992) Evolution and ecology of influenza A viruses. Microbiol Rev 56(1):152–179
Wetlands International (2012) Waterbird population estimates, 5th edn. Summary report. Wetlands International, Wageningen. ISBN: 978-90-5882-000-6
White CL, Ip HS, Meteyer CU, Walsh DP, Hall JS, Carstensen M, Wolf PC (2014) Spatial and temporal patterns of avian paramyxovirus-1 outbreaks in double-crested cormorants (Phalacrocorax auritus) in the USA. J Wildl Dis. doi:10.7589/2014-05-132
Wilcox BR, Knutsen GA, Berdeen J, Goekjian VH, Poulson R, Goyal S, Sreevatsan S, Cardona C, Berghaus V, Swayne DE, Yabsley MJ, Stallknecht DE (2011) Influenza A viruses in ducks in Northwestern Minnesota: fine scale spatial and temporal variation in prevalence and subtype diversity. PLoS One 6:e24010. doi:10.1371/journal.pone.0024010
Wille M, Robertson GJ, Whitney H, Bishop MA, Runstadler J, Lang AS (2011a) Extensive geographic mosaicism in avian influenza viruses from gulls in the northern hemisphere. PLoS One 6:e20664. doi:10.1371/journal.pone.0020664
Wille M, Robertson GJ, Whitney H, Ojkic D, Lang AS (2011b) Reassortment of American and Eurasian genes in an influenza A virus isolated from a great black-backed gull (Larus marinus), a species demonstrated to move between these regions. Arch Virol 156(1):107–115. doi:10.1007/s00705-010-0839-1
Wille M, Tolf C, Avril A, Latorre-Margalef N, Wallerstrom S, Olsen B, Waldenstrom J (2013) Frequency and patterns of reassortment in natural influenza A virus infection in a reservoir host. Virology 443(1):150–160. doi:10.1016/j.virol.2013.05.004
Wille M (2015) Viruses on the wing: evolution and dynamics of influenza A virus in the Mallard reservoir. Doctoral thesis, Linnaeus University Press. ISBN: 978-91-87925-56-6
Wille M, Latorre-Margalef N, Tolf C, Stallknecht DE, Waldenström J (2016) No evidence for homosubtypic immunity to influenza H3 in mallards following vaccination in a natural experimental system. Mol Ecol. doi:10.1111/mec.13967
Winker K, Gibson DD (2010) The Asia-to-America influx of avian influenza wild bird hosts is large. Avian Dis 54:477–482
Winker K, Spackman E, Swayne DE (2008) Rarity of influenza A virus in spring shorebirds, Southern Alaska. Emerg Infect Dis 14:1314–1316
Wobeser G (1992) Avian cholera and waterfowl biology. J Wildl Dis 28(4):674–682
Wobeser GA (1997) Diseases of wild waterfowl, 2nd edn. Plenum Press, New York
Wolfe ND, Dunavan CP, Diamond J (2007) Origins of major human infectious diseases. Nature 447(7142):279–283. doi:10.1038/nature05775
Woo PC, Lau SK, Lam CS, Lai KK, Huang Y, Lee P, Luk GS, Dyrting KC, Chan KH, Yuen KY (2009) Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J Virol 83(2):908–917. doi:10.1128/JVI.01977-08
Woo PC, Lau SK, Huang Y, Lam CS, Poon RW, Tsoi HW, Lee P, Tse H, Chan AS, Luk G, Chan KH, Yuen KY (2010) Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds, and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in the family Picornaviridae. J Gen Virol 91(Pt 10):2433–2448. doi:10.1099/vir.0.021717-0
Woolhouse ME, Gowtage-Sequeria S (2005) Host range and emerging and reemerging pathogens. Emerg Infect Dis 11(12):1842–1847. doi:10.3201/eid1112.050997
Worobey M, Han GZ, Rambaut A (2014) A synchronized global sweep of the internal genes of modern avian influenza virus. Nature 508(7495):254–257. doi:10.1038/nature13016
Wu H, Peng X, Xu L, Jin C, Cheng L, Lu X, Xie T, Yao H, Wu N (2014a) Novel reassortant influenza A(H5N8) viruses in domestic ducks, eastern China. Emerg Infect Dis 20(8):1315–1318. doi:10.3201/eid2008.140339
Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF (2014b) Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol 22(4):183–191. doi:10.1016/j.tim.2014.01.010
Yu K, Li Y, Han H, Song M, Ma X, Liu C, Huang B, Li F (2014) Complete genome sequence of an avian reovirus isolated from wild mallard ducks in china. Genome Announc 2(5). doi:10.1128/genomeA.00813-14
Yun T, Yu B, Ni Z, Ye W, Chen L, Hua J, Zhang C (2013) Isolation and genomic characterization of a classical Muscovy duck reovirus isolated in Zhejiang, China. Infect Genet Evol 20:444–453. doi:10.1016/j.meegid.2013.10.004
Zhang Y, Guo D, Geng H, Liu M, Hu Q, Wang J, Tong G, Kong X, Liu N, Liu C (2007) Characterization of M-class genome segments of Muscovy duck reovirus S14. Virus Res 125(1):42–53. doi:10.1016/j.virusres.2006.12.004
Zohari S, Neimanis A, Harkonen T, Moraeus C, Valarcher JF (2014) Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill 19(46)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Compliance with Ethical Standards
Funding: This study was funded by the Swedish Research Council (2013-7510, 2015-03877).
Conflict of Interest: Michelle Wille declares that he/she has no conflict of interest. Neus Latorre-Margalef declares that he/she has no conflict of interest. Jonas Waldenstrm declares that he/she has no conflict of interest.
Ethical approval: All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wille, M., Latorre-Margalef, N., Waldenström, J. (2017). Of Ducks and Men: Ecology and Evolution of a Zoonotic Pathogen in a Wild Reservoir Host. In: Hurst, C. (eds) Modeling the Transmission and Prevention of Infectious Disease. Advances in Environmental Microbiology, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-60616-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-60616-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-60614-9
Online ISBN: 978-3-319-60616-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)