Abstract
In the central nervous system (CNS), astrocytes are the most numerous among glial cells and have diverse physiological functions. These cells also play an important role in many CNS disorders and pathologies. Pituitary adenylate cyclase activating polypeptide (PACAP) is abundantly expressed in the CNS, acting as a neuroprotectant against various neurological threats. PACAP also has been studied as an astrocytic regulator from diversified aspects. PACAP receptor expression has dynamically changed in pathological condition. In this chapter, we summarize the expression and function of PACAP and PACAP receptors in astrocytes and astrocytic tumors, and discuss the role of PACAP in physiological and pathological conditions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–91.
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol (Berl). 2010;119:7–35.
Sovrea AS, Bosca AB. Astrocytes reassessment—an evolving concept part one: embryology, biology, morphology and reactivity. J Mol Psychiatry. 2013;1:18.
Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. 2015;7(6).
Jha MK, Suk K. Management of glia-mediated neuroinflammation and related patents. Recent Pat Inflamm Allergy Drug Discov. 2014;8:118–24.
Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–95.
Caruso C, Carniglia L, Durand D, Scimonelli TN, Lasaga M. Astrocytes: new targets of melanocortin 4 receptor actions. J Mol Endocrinol. 2013;51:R33–50.
Nakamachi T, Farkas J, Watanabe J, Ohtaki H, Dohi K, Arata S, et al. Role of PACAP in neural stem/progenitor cell and astrocyte—from neural development to neural repair. Curr Pharm Des. 2011;17:973–84.
Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, et al. Role of PACAP and VIP in astroglial functions. Peptides. 2007;28:1753–60.
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74.
Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88.
Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–70.
Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–70.
Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17.
Tatsuno I, Gottschall PE, Koves K, Arimura A. Demonstration of specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat astrocytes. Biochem Biophys Res Commun. 1990;168:1027–33.
Tatsuno I, Gottschall PE, Arimura A. Specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat cultured astrocytes: molecular identification and interaction with vasoactive intestinal peptide (VIP). Peptides. 1991;12:617–21.
Grimaldi M, Cavallaro S. Functional and molecular diversity of PACAP/VIP receptors in cortical neurons and type I astrocytes. Eur J Neurosci. 1999;11:2767–72.
Grimaldi M, Cavallaro S. Expression and coupling of PACAP/VIP receptors in cortical neurons and type I astrocytes. Ann N Y Acad Sci. 2000;921:312–6.
Hashimoto H, Kunugi A, Arakawa N, Shintani N, Fujita T, Kasai A, et al. Possible involvement of a cyclic AMP-dependent mechanism in PACAP-induced proliferation and ERK activation in astrocytes. Biochem Biophys Res Commun. 2003;311:337–43.
Chi-Wei L, Chang SL, Weng CF. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulates the expression of PACAP in cultured tilapia astrocytes. Exp Biol Med. 2007;232:262–76.
Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, et al. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413.
Nakamachi T, Ohtaki H, Yofu S, Dohi K, Watanabe J, Hayashi D, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul Pept. 2008;145:88–95.
Nakamachi T, Nakamura K, Oshida K, Kagami N, Mori H, Watanabe J, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates proliferation of reactive astrocytes in vitro. J Mol Neurosci. 2011;43:16–21.
Suzuki R, Arata S, Nakajo S, Ikenaka K, Kikuyama S, Shioda S. Expression of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC1-R) in reactive astrocytes. Brain Res Mol Brain Res. 2003;115:10–20.
Tsuchikawa D, Nakamachi T, Tsuchida M, Wada Y, Hori M, Farkas J, et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci. 2012;48:508–17.
Nakamachi T, Tsuchida M, Kagami N, Yofu S, Wada Y, Hori M, et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci. 2012;48:518–25.
Nakamachi T, Farkas J, Kagami N, Wada Y, Hori M, Tsuchikawa D, et al. Expression and distribution of pituitary adenylate cyclase-activating polypeptide receptor in reactive astrocytes induced by global brain ischemia in mice. Acta Neurochir Suppl. 2013;118:55–9.
Nishimoto M, Miyakawa H, Wada K, Furuta A. Activation of the VIP/VPAC2 system induces reactive astrocytosis associated with increased expression of glutamate transporters. Brain Res. 2011;1383:43–53.
Tatsuno I, Morio H, Tanaka T, Uchida D, Hirai A, Tamura Y, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a regulator of astrocytes: PACAP stimulates proliferation and production of interleukin 6 (IL-6), but not nerve growth factor (NGF), in cultured rat astrocyte. Ann N Y Acad Sci. 1996;805:482–8.
Moroo I, Tatsuno I, Uchida D, Tanaka T, Saito J, Saito Y, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes. Brain Res. 1998;795:191–6.
Nakatani M, Seki T, Shinohara Y, Taki C, Nishimura S, Takaki A, et al. Pituitary adenylate cyclase-activating peptide (PACAP) stimulates production of interleukin-6 in rat Muller cells. Peptides. 2006;27:1871–6.
Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP. VIP PHI Pharmacol Rep. 2005;57:307–20.
Ohtaki H, Nakamachi T, Dohi K, Shioda S. Role of PACAP in ischemic neural death. J Mol Neurosci. 2008;36:16–25.
Nakamachi T, Ohtaki H, Yofu S, Dohi K, Watanabe J, Mori H, et al. Endogenous pituitary adenylate cyclase activating polypeptide is involved in suppression of edema in the ischemic brain. Acta Neurochir Suppl. 2010;106:43–6.
Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–89.
Endo K, Nakamachi T, Seki T, Kagami N, Wada Y, Nakamura K, et al. Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci. 2011;43:22–9.
Masmoudi-Kouki O, Douiri S, Hamdi Y, Kaddour H, Bahdoudi S, Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J Neurochem. 2011;117:403–11.
Jozwiak-Bebenista M, Kowalczyk E, Nowak JZ. The cyclic AMP effects and neuroprotective activities of PACAP and VIP in cultured astrocytes and neurons exposed to oxygen-glucose deprivation. Pharmacol Rep. 2015;67:332–8.
Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, et al. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–60.
Kong LY, Maderdrut JL, Jeohn GH, Hong JS. Reduction of lipopolysaccharide-induced neurotoxicity in mixed cortical neuron/glia cultures by femtomolar concentrations of pituitary adenylate cyclase-activating polypeptide. Neuroscience. 1999;91:493–500.
Li M, David C, Kikuta T, Somogyvari-Vigh A, Arimura A. Signaling cascades involved in neuroprotection by subpicomolar pituitary adenylate cyclase-activating polypeptide 38. J Mol Neurosci. 2005;27:91–105.
Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–72.
Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–9.
Tatsuno I, Somogyvari-Vigh A, Mizuno K, Gottschall PE, Hidaka H, Arimura A. Neuropeptide regulation of interleukin-6 production from the pituitary: stimulation by pituitary adenylate cyclase activating polypeptide and calcitonin gene-related peptide. Endocrinology. 1991;129:1797–804.
Nagashima AC, Giacomini D, Castro CP, Pereda MP, Renner U, Stalla GK, et al. Transcriptional regulation of interleukin-6 in pituitary folliculo-stellate TtT/GF cells. Mol Cell Endocrinol. 2003;201:47–56.
Matsumoto H, Koyama C, Sawada T, Koike K, Hirota K, Miyake A, et al. Pituitary folliculo-stellate-like cell line (TtT/GF) responds to novel hypophysiotropic peptide (pituitary adenylate cyclase-activating peptide), showing increased adenosine 3′,5′-monophosphate and interleukin-6 secretion and cell proliferation. Endocrinology. 1993;133:2150–5.
Gottschall PE, Tatsuno I, Arimura A. Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP). Brain Res. 1994;637:197–203.
Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–93.
Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–6.
Brenneman DE, Hauser JM, Spong C, Phillips TM. Chemokine release is associated with the protective action of PACAP-38 against HIV envelope protein neurotoxicity. Neuropeptides. 2002;36:271–80.
Sanchez A, Tripathy D, Grammas P. RANTES release contributes to the protective action of PACAP38 against sodium nitroprusside in cortical neurons. Neuropeptides. 2009;43:315–20.
Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–93.
Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci. 2003;20:315–22.
Gozes I. Activity-dependent neuroprotective protein: from gene to drug candidate. Pharmacol Ther. 2007;114:146–54.
Gozes I. NAP (davunetide) provides functional and structural neuroprotection. Curr Pharm Des. 2011;17:1040–4.
Nakamachi T, Li M, Shioda S, Arimura A. Signaling involved in pituitary adenylate cyclase-activating polypeptide-stimulated ADNP expression. Peptides. 2006;27:1859–64.
Masmoudi O, Gandolfo P, Leprince J, Vaudry D, Fournier A, Patte-Mensah C, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates endozepine release from cultured rat astrocytes via a PKA-dependent mechanism. FASEB J. 2003;17:17–27.
Masmoudi-Kouki O, Gandolfo P, Leprince J, Vaudry D, Pelletier G, Fournier A, et al. PACAP stimulates biosynthesis and release of endozepines from rat astrocytes. Ann N Y Acad Sci. 2006;1070:411–6.
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25.
Ahmed R, Oborski MJ, Hwang M, Lieberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res. 2014;6:149–70.
Robberecht P, Woussen-Colle MC, Vertongen P, De Neef P, Hou X, Salmon I, et al. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) receptors in human glial cell tumors. Peptides. 1994;15:661–5.
Vertongen P, Camby I, Darro F, Kiss R, Robberecht P. VIP and pituitary adenylate cyclase activating polypeptide (PACAP) have an antiproliferative effect on the T98G human glioblastoma cell line through interaction with VIP2 receptor. Neuropeptides. 1996;30:491–6.
Sharma A, Walters J, Gozes Y, Fridkin M, Brenneman D, Gozes I, et al. A vasoactive intestinal peptide antagonist inhibits the growth of glioblastoma cells. J Mol Neurosci. 2001;17:331–9.
Dufes C, Alleaume C, Montoni A, Olivier JC, Muller JM. Effects of the vasoactive intestinal peptide (VIP) and related peptides on glioblastoma cell growth in vitro. J Mol Neurosci. 2003;21:91–102.
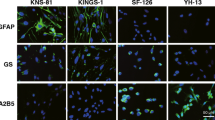
Nakamachi T, Sugiyama K, Watanabe J, Imai N, Kagami N, Hori M, et al. Comparison of expression and proliferative effect of pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors on human astrocytoma cell lines. J Mol Neurosci. 2014;54:388–94.
Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63:2–10.
Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45.
Rusnakova V, Honsa P, Dzamba D, Stahlberg A, Kubista M, Anderova M. Heterogeneity of astrocytes: from development to injury—single cell gene expression. PLoS One. 2013;8:e69734.
Acknowledgments
This work was supported by JSPS KAKENHI 23249079, 15K15670, 15H04394 and by a research grant from Toyama First Bank Scholarship Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nakamachi, T. (2016). Role of PACAP in Astrocytes and Astrocytic Tumors. In: Reglodi, D., Tamas, A. (eds) Pituitary Adenylate Cyclase Activating Polypeptide — PACAP. Current Topics in Neurotoxicity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-35135-3_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-35135-3_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-35133-9
Online ISBN: 978-3-319-35135-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)