Abstract
The diversity and the specialized connectivity and function of inhibitory cortical neurons have been the focus of intense research for many decades (Fishell and Rudy, Ann Rev Neurosci 34:535–567, 2011). Until recently, technical limitations have restricted the power of experiments that could be conducted in vivo. Nevertheless, in vitro studies identified dozens of distinct cortical inhibitory neuron types, each with unique chemical properties, intrinsic firing properties and connection specificity. And at the same time, post-mortem studies from human patients have demonstrated defects of inhibitory circuit markers in diseases such as schizophrenia (Curley and Lewis, J Physiol 590:715–724, 2012; Stan and Lewis, Curr Pharm Biotech 13:1557–1562, 2012; Lewis, Curr Opin Neurobiol 26:22–26, 2014). Together, these observations have led to the hypothesis that distinct types of inhibitory neurons play distinct functional roles in the dynamic regulation of brain states and in the context-dependent extraction of sensory information, cognitive function, and behavioral output—functions thought to be disrupted in disorders such as schizophrenia and autism.
Despite the wealth of evidence in support of this hypothesis, tools have only recently emerged to allow detailed studies of neural circuit mechanisms underlying in vivo dynamics and to implicate specific inhibitory cell types and connections in specific functions (Luo et al., Neuron 57:634–660, 2008). Now, rather than broadly surveying inhibitory neuron properties and connections in vitro, studies have begun to focus more deeply on the in vivo contributions of those inhibitory cell types that are genetically accessible and can therefore be interrogated with modern genetic tools for manipulating and monitoring activity of specific cell types.
Mouse lines that express Cre-recombinase selectively in three major, non-overlapping groups of inhibitory cortical neurons—Parvalbumin-expressing (PV), somatostatin-expressing (SST), and vasoactive intestinal peptide-expressing (VIP; Lee et al., J Neurosci 30:16796–16808, 2010; Xu et al. J Comp Neurol 518:389–404, 2010; Rudy et al., J Comp Neurol 518:389–404, 2011; Taniguchi et al., J Comp Neurol 518:389–404, 2011)—have allowed detailed studies of the connectivity and in vivo functional roles of these cell groups. Such studies have implicated PV inhibitory neurons in gain control (Atallah et al., Neuron 73:159–170, 2012; Lee et al., Nature 488:379–383, 2012; Nienborg et al., J Neurosci 33:11145–11154, 2013), SST interneurons in the suppression of lateral and feedback (top-down) interactions (Adesnik and Scanziani, Nature 464:1155–1160, 2010; Nienborg et al., J Neurosci 33:11145–11154, 2013), and VIP interneurons in the dynamic regulation of SST cells under the control of brain state-dependent neuromodulators (Kawaguchi, J Neurophysiol 78:1743–1747, 1997; Alitto and Dan, Front Syst Neurosci 6:79, 2012; Lee et al., Nat Neurosci 16:1662–1670, 2013; Pi et al., Nature 503:521–524, 2013; Polack et al., Nat Neurosci 16:1331–1339, 2013; Fu et al., Cell 156:1139–1152, 2014; Stryker, Cold Spring Harbor Symp Quant Biol 79:1–9, 2014; Zhang et al., Science 345:660–665, 2014).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Differences in Connectivity, Visual Responses and Functional Impact of PV Versus SST Interneurons
The in vivo functional role of any given neuron type is dictated by it sources of inputs, the way that it integrates those inputs, and the other neurons in the network to which it provides outputs. These differences result in measurable differences in visual receptive fields and differences in functional impact that can be assayed to understand how networks of neurons work together to generate perception and behavior.
Differential Outputs and Inputs
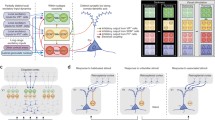
Among the first differences observed between SST and PV interneurons were morphological differences related to the locations of their synaptic contacts onto excitatory pyramidal neurons. The great majority of PV neurons are basket cells, so named because their axon terminals have the appearance of baskets. Basket cells make multiple, large synapses on the proximal dendrites and cell bodies of pyramidal neurons (Jones and Hendry 1984) and, therefore, even the connections originating from a single neuron may profoundly influence the activity of a recipient pyramid (Tamas et al. 2000, 2004). The typical basket cell expresses PV and is fast-spiking (FS; Cauli et al. 1997; Gonchar and Burkhalter 1997; Kawaguchi and Kubota 1997). FS basket cells are the most common inhibitory cell type and comprise about half of all cortical inhibitory neurons. In contrast, SST interneurons are dendrite targeting and most are “Martinotti cells” (Kawaguchi and Kubota 1997; Wang et al. 2004; Xu and Callaway 2009). Martinotti cells are regular spiking and have axons that typically extend to layer 1, where they make connections onto the apical dendrites of pyramidal cells. This observation led to the suggestion that Martinotti cells selectively inhibit excitatory inputs that impinge on the apical tufts of pyramids. This hypothesis was first suggested with respect to Martinotti cells in the hippocampus (Somogyi et al. 1998) where they might selectively influence input from the perforant path versus the Schaeffer collaterals that selectively target the corresponding regions of CA1 pyramidal neurons. In the cortex, however, there is a much more diverse population of excitatory neurons of both pyramidal and spiny stellate morphology, situated across multiple cortical layers. There are also diverse sources of excitatory input onto apical dendritic tufts. Nevertheless, prominent sources of input to the apical tufts of neocortical pyramidal neurons include feedback connections from other cortical areas as well as local lateral axonal arbors of other pyramidal neurons. These observations contributed to early hypotheses that SST-expressing Martinotti cells might preferentially regulate feedback and lateral influences, as tested in experiments described below. Taken together, even the earliest observations of differences in the outputs of PV versus SST interneurons suggested that PV-expressing basket cells have a global influence on pyramidal neurons whereas SST-expressing Martinotti cells have a more selective influence on inputs to apical dendrites.
PV and SST cells also differ in their sources of input. While PV cells in superficial cortical layers receive strong feedforward excitatory input from layer 4, as well as recurrent connections from within layer 2/3, excitatory inputs to SST neurons are dominated by layer 2/3 (Dantzker and Callaway 2000; Xu and Callaway 2009; Adesnik et al. 2012). Further exploration of the excitatory inputs to SST neurons shows that they collect local input over a longer lateral extent than pyramidal neurons, but PV neurons were not explored (Adesnik et al. 2012). Inhibitory inputs to PV neurons arise predominantly from layer 2/3 whereas SST cells receive more balanced inhibition from layers 2/3 and 4 as well as layer 5 (Xu and Callaway 2009).
Visual Responses
Because PV neurons are FS cells, they can be identified during extracellular recordings in vivo. This ability has allowed their visual responses to be measured in diverse species, including ferrets, cats, and monkeys and mice. However, observations of the visual responses of SST neurons have only been described in mice, where they can be targeted genetically. In general, both PV and SST neurons appear to have visual responses that reflect the combined responses of their surrounding excitatory neurons. Thus, in species that have orientation columns, PV neurons have orientation-selective visual receptive fields. However, in mice, which lack orientation columns, PV and SST neurons are generally not orientation selective (Kerlin et al. 2010), apparently due to the indiscriminate collection of excitatory inputs from orientation-selective excitatory neurons that are tuned to a diversity of orientations. This connectional scheme fits with the functional role of PV neurons in providing gain control (see below). By monitoring the activity of its neighboring excitatory neurons, a PV neuron will increase its activity when the local network is most active and then provide feedback inhibition to keep activity levels under control. An important visual response feature that appears to be unique to SST cells, however, is that their visual responses increase in magnitude as the radius of a drifting grating stimulus increases (surround summation; Adesnik et al. 2012). More typical cells, including PV cells and excitatory neurons, instead display surround suppression (Adesnik et al. 2012). This feature of SST cells appears to be a consequence of the prominent lateral inputs that these neurons receive (see above) and has led to the hypothesis that they are the neurons responsible for generating surround suppression in other cell types (see further details below; Adesnik et al. 2012).
Functional Impact
The advent or Cre-driver mice combined with optogenetic tools has allowed direct tests of the functional impact of SST and PV neurons on visual responses. These experiments have demonstrated the importance of PV neurons in gain control and SST neurons in mediating surround influences. As expected, optogenetic activation of either inhibitory cell type results in decreased activity within the local cortical network, and such decreases are most prominent when PV cells are activated. For PV neurons, optogenetic activation decreases visual responses of excitatory neurons without altering their orientation tuning (Atallah et al. 2012; Lee et al. 2012). And when neurons are tested with visual stimuli of increasing radius, PV neuron activation mimics the effects of reducing the contrast of the visual stimulus; surround summation is observed rather than surround suppression (Nienborg et al. 2013). All of these effects indicate linear influences of PV cells and point to their role in controlling gain.
The functional influence of SST neurons, on the other hand, is non-linear. The classic feature of “end-stopping” in cortical neurons (Hubel and Wiesel 1968) is now better known as surround suppression. Here a visual stimulus presented in a zone that does not by itself generate any visual response in the subject neuron (outside the classical receptive field) can suppress the response to a stimulus within the classical receptive field. This interaction is clearly non-linear in that the response to the combined stimuli does not reflect the sum of the responses to the stimuli when they are presented separately. Optogenetic activation of SST neurons increases surround suppression in anesthetized animals where suppression is typically weak (Nienborg et al. 2013), and optogenetic inactivation of SST cells reduces surround suppression (Adesnik et al. 2012). Therefore, SST cells clearly contribute to the generation of surround suppression. It should be noted, however, that surround suppression is also present in the input to the cortex and this suppression is not prevented by cortical inactivation (Sceniak et al. 2006). Furthermore, inactivation of SST cells does not completely eliminate surround suppression of cortical neurons (Adesnik et al. 2012). Thus SST cells are likely responsible for cortical contributions to surround suppression but cannot be responsible for the suppression observed in the LGN input.
Calretinin (CR) and VIP-Expressing Interneurons Target SST Interneurons
Historically, the first study to implicate a specific type of cortical inhibitory neuron in local dis-inhibition was published by Meskenaite in 1997. This manuscript combined electron microscopy (EM) and antibody staining to show that CR-positive axon terminals in layer 2/3 of monkey V1 made 81 % of their contacts onto GABAergic neurons , but in layer 5 only 20 % of the contacts were onto GABAergic neurons. The remaining 80 % of contacts in layer 5 were onto pyramidal neurons, where they formed strong basket-like synapses. Furthermore, these contacts appeared to be biased toward large layer 5 pyramids (that project sub-cortically and lack local projections to layer 2/3) rather than small pyramids (that make dense recurrent projections to layer 2/3 and lack extrinsic projections in primate V1; Callaway and Wiser 1996). Meskenaite suggested that “the CR-immunoreactive neurons appear to have a dual function of disinhibiting superficial layer neurons and inhibiting pyramidal output neurons in the deep layers.” Meskenaite’s findings were closely followed by analogous EM experiments in the rat visual cortex, showing a similar trend for CR+ axon terminals targeting inhibitory neurons in layer 2/3 and pyramids in layer 5 (Gonchar and Burkhalter 1999).
In view of this evidence, why is it that recent studies of disinhibition in layer 2/3 of mouse cortex have focused on VIP inhibitory neurons rather than CR neurons? Prior to the emergence of the mouse as the most prevalent rodent model, studies in rats had shown that PV, SST, and CR neurons make up three distinct and non-overlapping cell groups in that species (Kawaguchi and Kondo 2002). Similar antibody-labeling studies conducted in mice revealed that there was substantial overlap between CR and SST expression (Xu et al. 2006), but that there was no comparable overlap between VIP and SST (Xu et al. 2010). Thus, when Cre driver lines became available to separately target gene expression to PV, SST, VIP, or CR neurons (Taniguchi et al. 2011), VIP was favored over CR because of the ability to target a population that was separate from PV or SST neurons. Studies of VIP neurons have so far proceeded without concern for the known diversity of VIP cell types, and the grouping of these cells into a monolithic population has appeared to be justified by the striking differences in the connectivity and functional impact of these cells when compared to PV or SST cells (Lee et al. 2013; Pfeffer et al. 2013).
However, it was already predictable from the published literature (Xu et al. 2006; Caputi et al. 2009) that the CR-expressing subpopulation of VIP neurons preferentially targets SST cells in layer 2/3 of mouse. Caputi et al. (2009) produced and studied a mouse line in which GFP was expressed in CR neurons. They conducted extensive paired recordings to evaluate the rate of connectivity between numerous cell types, primarily focusing on the GFP-positive CR neurons. They noted that there were two types of CR/GFP neurons in their material, bipolar and multipolar. Remarkably, they found that the bipolar neurons had an astounding 76.4 % rate of connectivity (13/17 pairs) onto the multipolar neurons in layer 2/3 but made connections far less frequently onto layer 2/3 pyramids (11.6 %, 7 of 60). Connections from layer 2/3 to layer 5 were not assessed in this study. While Caputi et al. appeared not to appreciate it at the time, reporting the most salient observations only in a table, the published literature clearly showed that their multipolar GFP neurons are SST-expressing Martinotti cells, whereas their bipolar cells are CR positive and SST negative (Xu et al. 2006). Thus, it is apparent that mouse CR bipolar cells preferentially target SST neurons in layer 2/3. In this context, it is not entirely surprising that later studies systematically investigating the connectivity of PV, SST and VIP neurons found strong connections from VIP neurons onto SST neurons in layer 2/3 and not in layer 5 (Lee et al. 2013; Pfeffer et al. 2013). It remains unclear whether this is a feature of all VIP interneurons or only of the CR-expressing subpopulation. Nevertheless, it would be surprising if there were not differences in the connectivity and function of CR-positive versus CR-negative VIP interneurons.
Functional Impact of VIP Interneurons
Based on the preferential connections of VIP neurons onto SST neurons, it is natural to predict that VIP neurons selectively regulate the impact of SST cells. For example, conditions that increase VIP neuron activity might be expected to inhibit SST cells, allowing greater influence from the lateral and feedback excitatory connections that target the apical tufts of pyramidal neurons. On the other hand, if the population of VIP neurons is diverse, the effects of manipulating these cells might be less predictable.
Recent studies have demonstrated that VIP neurons in mouse visual cortex are engaged during locomotion, apparently through a mechanism involving locomotion-induced increases in cholinergic input to the cortex (Fu et al. 2014; Reimer et al. 2014; Stryker 2014). However, rather than simply decreasing the activity of SST neurons, locomotion appears to have diverse effects (Polack et al. 2013; Reimer et al. 2014). These discrepancies might be related to the diversity of VIP neurons or could be attributable to other unknown differences in experimental methods. It is likely, however, that further dissection of the VIP neuron population using intersectional genetic methods will help to resolve these issues.
References
Adesnik H, Scanziani M (2010) Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464:1155–1160
Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M (2012) A neural circuit for spatial summation in visual cortex. Nature 490:226–231
Alitto HJ, Dan Y (2012) Cell-type-specific modulation of neocortical activity by basal forebrain input. Front Syst Neurosci 6:79
Atallah BV, Bruns W, Carandini M, Scanziani M (2012) Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73:159–170
Callaway EM, Wiser AK (1996) Contributions of individual layer 2-5 spiny neurons to local circuits in macaque primary visual cortex. Vis Neurosci 13:907–922
Caputi A, Rozov A, Blatow M, Monyer H (2009) Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex 19:1345–1359
Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J (1997) Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17:3894–3906
Curley AA, Lewis DA (2012) Cortical basket cell dysfunction in schizophrenia. J Physiol 590:715–724
Dantzker JL, Callaway EM (2000) Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci 3:701–707
Fishell G, Rudy B (2011) Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci 34:535–567
Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP (2014) A cortical circuit for gain control by behavioral state. Cell 156:1139–1152
Gonchar Y, Burkhalter A (1997) Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7:347–358
Gonchar Y, Burkhalter A (1999) Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex. Cereb Cortex 9:683–696
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195:215–243
Jones EG, Hendry SHC (1984) Basket cells. In: Peters A, Jones EG (eds) Cerebral cortex. Plenum, New York, pp 309–336
Kawaguchi Y (1997) Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol 78:1743–1747
Kawaguchi Y, Kondo S (2002) Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31:277–287
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7:476–486
Kerlin AM, Andermann ML, Berezovskii VK, Reid RC (2010) Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67:858–871
Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30:16796–16808
Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y (2012) Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488:379–383
Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B (2013) A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16:1662–1670
Lewis DA (2014) Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 26:22–26
Luo L, Callaway EM, Svoboda K (2008) Genetic dissection of neural circuits. Neuron 57:634–660
Meskenaite V (1997) Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol 379:113–132
Nienborg H, Hasenstaub A, Nauhaus I, Taniguchi H, Huang ZJ, Callaway EM (2013) Contrast dependence and differential contributions from somatostatin- and parvalbumin-expressing neurons to spatial integration in mouse V1. J Neurosci 33:11145–11154
Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16:1068–1076
Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503:521–524
Polack PO, Friedman J, Golshani P (2013) Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16:1331–1339
Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS (2014) Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84:355–362
Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100 % of neocortical GABAergic neurons. Dev Neurobiol 71:45–61
Sceniak MP, Chatterjee S, Callaway EM (2006) Visual spatial summation in macaque geniculocortical afferents. J Neurophysiol 96:3474–3484
Somogyi P, Tamas G, Lujan R, Buhl EH (1998) Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26:113–135
Stan AD, Lewis DA (2012) Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol 13:1557–1562
Stryker MP (2014) A neural circuit that controls cortical state, plasticity, and the gain of sensory responses in mouse. Cold Spring Harb Symp Quant Biol 79:1–9
Tamas G, Buhl EH, Lorincz A, Somogyi P (2000) Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci 3:366–371
Tamas G, Szabadics J, Lorincz A, Somogyi P (2004) Input and frequency-specific entrainment of postsynaptic firing by IPSPs of perisomatic or dendritic origin. Eur J Neurosci 20:2681–2690
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013
Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H (2004) Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol 561:65–90
Xu X, Callaway EM (2009) Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci 29:70–85
Xu X, Roby KD, Callaway EM (2006) Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499:144–160
Xu X, Roby KD, Callaway EM (2010) Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518:389–404
Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y (2014) Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345:660–665
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution-Noncommercial 2.5 License (http://creativecommons.org/licenses/by-nc/2.5/) which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
The images or other third party material in this chapter are included in the work’s Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work’s Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Callaway, E.M. (2016). Inhibitory Cell Types, Circuits and Receptive Fields in Mouse Visual Cortex. In: Kennedy, H., Van Essen, D., Christen, Y. (eds) Micro-, Meso- and Macro-Connectomics of the Brain. Research and Perspectives in Neurosciences. Springer, Cham. https://doi.org/10.1007/978-3-319-27777-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-27777-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27776-9
Online ISBN: 978-3-319-27777-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)