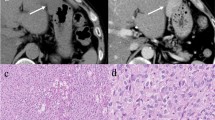
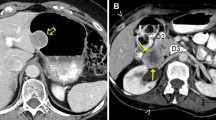
Abstract
Gastrointestinal stromal tumor (GIST), although rare, is the most common mesenchymal gastrointestinal neoplasm. This chapter will review the epidemiology, environmental factors, genetic predisposition, and underlying biomolecular changes of the disease. The staging of GIST as well as the roles of conventional diagnostic imaging and nuclear imaging in this staging will be reviewed. Finally, the efficacy of these modalities in assessing response to the various treatments described in the chapter and in the long-term surveillance for disease recurrence will be addressed.
Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AFIP:
-
Armed Forces Institute of Pathology
- AJCC:
-
American Joint Committee on Cancer
- BRAF:
-
Gene encoding for the B-Raf protein, a serine/threonine-protein kinase; the gene is also known as the proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B
- CSF-1R:
-
Colony-stimulating factor 1R
- CT:
-
X-ray computed tomography
- DECT:
-
Dual-energy CT
- EORTC:
-
European Organization for Research and Treatment of Cancer
- EUS:
-
Endoscopic ultrasound
- [18F]FDG:
-
2-deoxy-2-[18F]fluoro-D-glucose
- FDA:
-
United States Food and Drug Administration
- GIST:
-
Gastrointestinal stromal tumor
- GLUT4:
-
Glucose transporter 4
- HPF:
-
High-power fields
- HU:
-
Hounsfield units
- IRA:
-
Iodine-related attenuation
- KIT:
-
A proto-oncogene encoding for tyrosine-protein kinase Kit (or CD117), also known as mast/stem cell growth factor receptor (SCFR)
- M:
-
Metastasis status according to the AJCC/UICC TNM staging system
- MRI:
-
Magnetic resonance imaging
- N:
-
Lymph node status according to the AJCC/UICC TNM staging system
- NCCN:
-
National Comprehensive Cancer Network
- NF1:
-
Neurofibromatosis type 1
- PDGFR:
-
Platelet-derived growth factor receptor
- PDGFRA:
-
Platelet-derived growth factor receptor alpha
- PET:
-
Positron emission tomography
- PET/CT:
-
Positron emission tomography/Computed tomography
- RECIST:
-
Response evaluation criteria in solid tumors
- RET:
-
A proto-oncogene encoding for a receptor tyrosine kinase for extracellular signaling molecules (from “rearranged during transfection”)
- RTK:
-
Receptor tyrosine kinase
- SCF:
-
Stem cell factor
- SDH:
-
Succinate dehydrogenase
- SURE:
-
Sunitinib alternating with regorafenib
- SUVmax:
-
Standardized uptake value at point of maximum
- SUV:
-
Standardized uptake value
- TKI:
-
Tyrosine kinase inhibitor
- TNM:
-
AJCC/UICC staging system based on parameters “T” (tumor status), “N” (lymph node status), and “M” (distant metastasis status)
- T:
-
Tumor status according to the AJCC/UICC TNM staging system
- UICC:
-
International Union Against Cancer (Union Internationale Contre le Cancer)
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30(10):1213–20.
Miettinen M, Lasota J. Gastrointestinal stromal tumors – definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–78.
Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomark Prev. 2014;24(1):298–302.
Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46.
American Cancer Society. Key Statistics for Gastrointestinal Stromal Tumors [updated December 1, 2019], [Accessed March 26, 2021]. Available from: https://www.cancer.org/cancer/gastrointestinal-stromal-tumor/about/key-statistics.html.
Chetty R, Serra S. Molecular and morphological correlation in gastrointestinal stromal tumours (GISTs): an update and primer. J Clin Pathol. 2016;69(9):754–60.
Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45(3):357–65.
Baykara M, Akkus M, Yildiz R, Gonul II, Dursun A, Coskun U, et al. Survivin expression and its potential clinical significance in gastrointestinal stromal sarcoma. Int Immunopharmacol. 2011;11(12):2227–31.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83.
Morgan J, Raut CR, Duensing A, Keedy VL. Epidemiology, classification, clinical presentation, prognostic features, and diagnostic work-up of gastrointestinal stromal tumors (GIST) 2020 [updated July 14, 2020. Available from: https://www.uptodate.com/contents/epidemiology-classification-clinical-presentation-prognostic-features-and-diagnostic-work-up-of-gastrointestinal-stromal-tumors-gist.
Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46.
Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–96.
Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14(10):3204–15.
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–10.
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23): 4342–9.
Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29):4764–74.
Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012;136(5):483–9.
Martin J, Poveda A, Llombart-Bosch A, Ramos R, Lopez-Guerrero JA, Garcia del Muro J, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol. 2005;23(25):6190–8.
Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26(33):5352–9.
Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–90.
Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357–64.
Nannini M, Astolfi A, Urbini M, Indio V, Santini D, Heinrich MC, et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST). BMC Cancer. 2014;14:685.
Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30(1):90–6.
Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23(1):15–34. vii.
Janeway KA, Albritton KH, Van Den Abbeele AD, D’Amato GZ, Pedrazzoli P, Siena S, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52(7):767–71.
Bachet JB, Landi B, Laurent-Puig P, Italiano A, Le Cesne A, Levy P, et al. Diagnosis, prognosis and treatment of patients with gastrointestinal stromal tumour (GIST) and germline mutation of KIT exon 13. Eur J Cancer. 2013;49(11):2531–41.
Franck C, Rosania R, Franke S, Haybaeck J, Canbay A, Venerito M. The BRAF status may predict response to sorafenib in gastrointestinal stromal tumors resistant to imatinib, sunitinib, and regorafenib: case series and review of the literature. Digestion. 2019;99(2):179–84.
Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–25.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52–68.
Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30(4):477–89.
von Mehren M. The 2021 National Comprehensive Cancer Network (NCCN) guidelines for GISTs NCCN.org; 2021 [updated October 20, 2020]. Accessed March 26, 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf.
Jumniensuk C, Charoenpitakchai M. Gastrointestinal stromal tumor: clinicopathological characteristics and pathologic prognostic analysis. World J Surg Oncol. 2018;16(1):231.
Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9(9):3329–37.
De Smedt J, Van Kelst S, Boecxstaens V, Stas M, Bogaerts K, Vanderschueren D, et al. Vitamin D supplementation in cutaneous malignant melanoma outcome (ViDMe): a randomized controlled trial. BMC Cancer. 2017;17(1):562.
Yu MH, Lee JM, Baek JH, Han JK, Choi BI. MRI features of gastrointestinal stromal tumors. AJR Am J Roentgenol. 2014;203(5):980–91.
Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226(2):527–32.
Kim HC, Lee JM, Kim SH, Park SH, Lee JW, Lee M, et al. Small gastrointestinal stromal tumours with focal areas of low attenuation on CT: pathological correlation. Clin Radiol. 2005;60(3):384–8.
Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70(2):254–61.
Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol. 2009;6(6):363–71.
Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45(1):17–21.
Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 2013;13(1):3–18.
Edmonson JH, Marks RS, Buckner JC, Mahoney MR. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Investig. 2002;20(5–6):605–12.
Trent JC, Beach J, Burgess MA, Papadopolous N, Chen LL, Benjamin RS, et al. A two-arm phase II study of temozolomide in patients with advanced gastrointestinal stromal tumors and other soft tissue sarcomas. Cancer. 2003;98(12):2693–9.
Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136(4):383–9.
Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8(10):3034–8.
Demetri GD, Von Mehren M, Blanke CD, Van Den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99(1):42–7.
Cohen MH, Cortazar P, Justice R, Pazdur R. Approval summary: imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. Oncologist. 2010;15(3):300–7.
Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, et al. Adjuvant imatinib for high-risk GI stromal tumor: analysis of a randomized trial. J Clin Oncol. 2016;34(3):244–50.
Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 clinical trial. JAMA Oncol. 2018;4(12):e184060.
Sutent FDA. Package Insert [updated] 2006. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021938s033lbl.pdf
O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–605.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38.
George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–68.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302.
Heinrich MC, Jones RL, von Mehren M, Schoffski P, Serrano C, Kang YK, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21(7):935–46.
Gardino AK, Evans EK, Kim JL, Brooijmans N, Hodous BL, Wolf B, et al. Targeting kinases with precision. Mol Cell Oncol. 2018;5(3):e1435183.
Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–34.
Blay JY, von Mehren M. Ripretinib for advanced gastrointestinal stromal tumours – Authors’ reply. Lancet Oncol. 2020;21(9):e415.
Smith BD, Kaufman MD, Lu WP, Gupta A, Leary CB, Wise SC, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738–51 e9.
Montemurro M, Schoffski P, Reichardt P, Gelderblom H, Schutte J, Hartmann JT, et al. Nilotinib in the treatment of advanced gastrointestinal stromal tumours resistant to both imatinib and sunitinib. Eur J Cancer. 2009;45(13):2293–7.
Sawaki A, Nishida T, Doi T, Yamada Y, Komatsu Y, Kanda T, et al. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117(20):4633–41.
Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Investig New Drugs. 2012;30(6):2377–83.
Montemurro M, Gelderblom H, Bitz U, Schutte J, Blay JY, Joensuu H, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: a retrospective analysis. Eur J Cancer. 2013;49(5):1027–31.
Ganjoo KN, Villalobos VM, Kamaya A, Fisher GA, Butrynski JE, Morgan JA, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol. 2014;25(1):236–40.
Schoffski P, Mir O, Kasper B, Papai Z, Blay JY, Italiano A, et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ‘CaboGIST’. Eur J Cancer. 2020;134:62–74.
Trent JC, Wathen K, von Mehren M, Samuels BL, Staddon AP, Choy E, et al. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol. 2011;29(15_suppl):10006.
Schoffski P, Reichardt P, Blay JY, Dumez H, Morgan JA, Ray-Coquard I, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol. 2010;21(10):1990–8.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271–82.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Van den Abbeele AD, Badawi RD, Janicke M, Blanke C, Joensuu H, Roberts P, et al. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) [Abstract]. ASCO. 2001.
Van den Abbeele AD, Badawi RD. Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer. 2002;38(Suppl 5):S60–5.
Van den Abbeele AD. The lessons of GIST-PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13.
Van den Abbeele AD, Gatsonis C, de Vries DJ, Melenevsky Y, Szot-Barnes A, Yap JT, et al. ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med. 2012;53(4):567–74.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32(2):153–62.
Holdsworth CH, Badawi RD, Manola JB, Kijewski MF, Israel DA, Demetri GD, et al. CT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR Am J Roentgenol. 2007;189(6):W324–30.
Abdulkader I, Cameselle-Teijeiro J, Forteza J. Pathological changes related to Imatinib treatment in a patient with a metastatic gastrointestinal stromal tumour. Histopathology. 2005;46(4):470–2.
Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006;26(2):481–95.
Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH. Imatinib and beyond in gastrointestinal stromal tumors: A radiologist’s perspective. AJR Am J Roentgenol. 2013;201(4):801–10.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–9.
Meyer M, Hohenberger P, Apfaltrer P, Henzler T, Dinter DJ, Schoenberg SO, et al. CT-based response assessment of advanced gastrointestinal stromal tumor: dual energy CT provides a more predictive imaging biomarker of clinical benefit than RECIST or Choi criteria. Eur J Radiol. 2013;82(6):923–8.
Tang L, Zhang XP, Sun YS, Shen L, Li J, Qi LP, et al. Gastrointestinal stromal tumors treated with imatinib mesylate: apparent diffusion coefficient in the evaluation of therapy response in patients. Radiology. 2011;258(3):729–38.
Benjamin RS, Debiec-Rychter M, Le Cesne A, Sleijfer S, Demetri GD, Joensuu H, et al. Gastrointestinal stromal tumors II: medical oncology and tumor response assessment. Semin Oncol. 2009;36(4):302–11.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–34.
Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele AD, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235(3):892–8.
Howard SA, Krajewski KM, Thornton E, Jagannathan JP, O’Regan K, Cleary J, et al. Decade of molecular targeted therapy: abdominal manifestations of drug toxicities – what radiologists should know. AJR Am J Roentgenol. 2012;199(1):58–64.
Shinagare AB, Howard SA, Krajewski KM, Zukotynski KA, Jagannathan JP, Ramaiya NH. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol. 2012;199(6):1259–65.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry
Sakellis, C.G., Jacene, H.A., Van den Abbeele, A.D. (2022). Diagnostic Applications of Nuclear Medicine: Gastrointestinal Stromal Tumors. In: Volterrani, D., Erba, P.A., Strauss, H.W., Mariani, G., Larson, S.M. (eds) Nuclear Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-26067-9_15-2
Download citation
DOI: https://doi.org/10.1007/978-3-319-26067-9_15-2
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26067-9
Online ISBN: 978-3-319-26067-9
eBook Packages: Springer Reference MedicineReference Module Medicine
Publish with us
Chapter history
-
Latest
Diagnostic Applications of Nuclear Medicine: Gastrointestinal Stromal Tumors- Published:
- 09 August 2022
DOI: https://doi.org/10.1007/978-3-319-26067-9_15-2
-
Original
Diagnostic Applications of Nuclear Medicine: Gastrointestinal Stromal Tumors- Published:
- 21 October 2016
DOI: https://doi.org/10.1007/978-3-319-26067-9_15-1