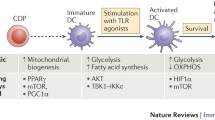
Abstract
Dendritic cells (DCs) are the sentinels of the immune system and play a critical role in stimulating immune responses against pathogens and maintaining immune homeostasis to harmless antigens. They can be found in all lymphoid and most non-lymphoid tissues, including mucosal surfaces, like the lung and the gut, where intricate networks of DCs are situated to sense potential harmful exposures. As such, DCs are among the first cells to come into contact with food bioactives in the gastrointestinal tract and thus are instrumental in shaping the immune system’s response to such exposures. Here we provide an overview of DC characteristics, with the emphasis on DCs in the mucosal immune system, and discuss in vitro/ex vivo DC culture settings that can be applied for in vitro testing of (food) compounds.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Origin
Paul Langerhans was the first to describe DCs in the nineteenth century, when he visualised dendritically shaped cells in the skin (Langerhans 1868). Ralph M. Steinman and Zanvil A. Cohn identified “dendritic cells” as a specific group of white blood cells (Steinman and Cohn 1973), and it was not until 1998 that the central role of DCs as professional antigen presenting cells for the induction of adaptive immunity was defined (Banchereau and Steinman 1998). Over the last few decades the DC-field has developed extensively with enhanced knowledge on DC progenitors, distinctive subsets, and their specific functions depending on the tissues where they reside.
2 Features and Mechanisms
DCs continuously sample antigens from their environment by phagocytosis, receptor-mediated endocytosis, and pinocytosis. After uptake of antigens, DCs migrate to the draining lymph node. During migration they may mature and upregulate co-stimulatory molecules like CD40 and CD86, depending on the maturation signals they have encountered. DCs are equipped with unique pattern recognition receptors (PRRs) for which the ligands may activate or inhibit DC maturation and facilitate antigen recognition, uptake and processing. The extensive sets of PRRs (e.g. Toll-like receptors (TLRs), C-type lectins (CLRs), Nod-like receptors (NLRs) and Retinoic acid induced gene-based (RIG)-I like receptors (RLRs)) are expressed on the cell surface or in intracellular compartments, like endosomes or in the cytoplasm to enable recognition of intra- and extracellular exposures.
Dendritic cells are instrumental for the activation of naïve T cells or reactivation of T cells from the memory pool (McLellan et al. 1996). T cell activation requires antigen presentation in MHC class I (for CD8+ T cell) or II (for CD4+ T cells) molecules (signal 1), in combination with expression of co-stimulatory molecules (signal 2). Cytokines provide a third signal and induce T cell differentiation and effector functions. Depending on the cytokine environment provided by DCs, CD4+ T cells differentiate into Thelper1 (Th1) via IL-12, Th2 via IL-4, or Th17 cells via IL-6 (Kapsenberg 2003; Bettelli et al. 2008). In addition, Th cells can also differentiate into regulatory phenotypes (Tregs, Th3 and Tr1) that dampen immune responses (Sakaguchi et al. 2006). On the other hand, DCs instruct CD8+ T cells, which recognize MHC class I (cross)presented antigens, to develop into cytotoxic lymphocytes (CTLs) (Bhardwaj et al. 1994).
The responsiveness of DCs in terms of expression of co-stimulatory/regulatory molecules and cytokine profiles may thus provide insight into the immune modulating properties of food bioactives. Here, we provide an overview of the different DC subsets in blood, and more specifically in the gastrointestinal tract (Fig. 17.1). Then we will provide information on the in vitro and ex vivo possibilities to study effects of food bioactives on DCs.
2.1 DC Subsets
To study immune modulatory effect of bioactives on DCs, different models systems may be considered, e.g. DC cell lines, in vitro-generated DCs, or directly isolated primary DCs, each with their own advantages and disadvantages. It is important to realize that different subsets of DCs exist in peripheral blood and (non)lymphoid tissues and that the differentiation and function of each of these subsets is highly dependent on the interacting cells and mediators in the local microenvironment. So, while this chapter provides experimental settings to study the direct effects of food bioactives on DCs, one should always consider the possible indirect effect via stimulation of the bioactives on for instance epithelial cells, and that different DC subsets may respond differently to the same exposure.
2.1.1 Blood DCs
DCs can be divided in conventional DCs (cDCs) and plasmacytoid DCs (pDCs). In human blood, both cDCs and pDCs can be phenotypically characterized by the lack of lineage marker expression (CD3, CD14, CD19, CD56) and a high expression of HLA-DR. The circulating cDCs are CD11c+ and are divided by the expression of CD1c (BDCA1) or CD141 (BDCA3). Human pDCs lack expression of CD11c and are discriminated by the expression of BDCA2, BDCA4 and/or CD123. CD16+ MHC class II+ cells cluster as a distinct population in the blood (Lindstedt et al. 2005) and have previously been associated with DC origins by some. However, CD16+ cells were recently assigned to the monocyte lineage (Robbins et al. 2008; Ziegler-Heitbrock et al. 2010), confirmed by transcript profiling comparing CD16+ cells with BDCA1+ DCs (Frankenberger et al. 2012).
2.1.2 Mucosal DCs
The mucosal immune system is highly specialized: it has to be able to avoid invasion of (commensal) bacteria, and tolerate their presence in the intestine. It should also induce tolerance to non-harmful food antigens and protect against potentially harmful pathogens, toxins and xenobiotics. All of this takes place over a single layer of highly specialized epithelial cells (Chapter 2).
The formulation of antigens determines the main site for antigen uptake in the intestinal tract. Considerable evidence suggests that the gut associated lymphoid tissues (GALT), such as Peyers Patches (PP) and isolated lymphoid follicles, are critical for handling of particulate antigens. The microfold cells (M cells), present in the GALT epithelium, are involved in actively transferring the particulate antigens from the gut lumen into the lymphoid areas. The role for GALT in the induction of oral tolerance to soluble antigens is not entirely clear. Normal oral tolerance could be induced in mice lacking PP. It was therefore suggested that the main function of the GALT is to control immune responses to commensal bacteria. Antigen uptake by DCs underlying the villus epithelium of the lamina propria (LP) in the small intestine has been shown to be crucial for induction of oral tolerance to soluble antigens. How the DCs are provided with the antigens over the epithelial barrier will strongly depend on the compound and is beyond the scope of this chapter. Since both the intestinal tract and its draining lymph nodes contain specific DC subsets we will briefly discuss them before providing detailed information of possible DC systems that could be used to mimic these DCs.
Intestinal DCs: Thorough comparative transcriptional and functional profiling in DCs isolated from a human and murine small intestine LP and peripheral blood showed three DC subsets within the CD45+lin−MHCII+ LP population based on the expression of CD103 and Sirpα (Watchmaker et al. 2014). The CD103+Sirpα− DCs (SP) were related to human blood BDCA3+ DCs, whereas CD103+Sirpα+ (DP) DCs expressed CD11b, CD207 (Langerin), CD209 and high levels of RALDH (coinciding with human blood CD1c+ DCs) and supported the induction of (FoxP3+) regulatory T cells. The CD103−Sirpa+ expressed CX3CR1, but lacked CD64 expression, and clustered with human monocytes indicating that they may have developed from monocytes recruited in response to gut inflammation. Most of these cells are located deeper into the LP when compared to the network of phagocytic cells that is located right beneath the epithelial cells. These phagocytic cells express CD45, HLA-DR, CD14, CD64 and high levels of CX3CR1, and since these cells also do not migrate to the lymph nodes, they have been depicted as intestinal macrophages (Rivollier et al. 2012; Bain et al. 2012; Tamoutounour et al. 2012; Mann et al. 2013).
Mesenteric lymph node DCs: Soluble food bioactives may also be directly available for internalization by DCs in the draining lymph nodes via the conduit system (Gretz et al. 1997; Anderson and Shaw 2005; Sixt et al. 2005; Roozendaal et al. 2009). The mesenteric lymph node DCs are a mixture of cells found in peripheral blood as well as the LP. As such, peripheral blood DCs and their CD34-derived counterparts could represent these lymph node DCs.
2.1.3 Monocyte-Derived DCs
Monocyte-derived (mo)DCs are rare under steady-state conditions in both blood and peripheral tissues. They can be found in increased numbers in vivo under inflammatory conditions. The ability to culture moDCs from monocytes boosted their popularity as a model to study human DC biology (Sallusto and Lanzavecchia 1994). The gene profile of in vitro-generated MoDCs is enriched for genes also found in in vivo occurring moDC (Segura et al. 2013) and also emphasizes the monocyte origin (not DC origin) with acquired DC features.
3 General Protocols
Primary DCs can be isolated from blood, and lymphoid or peripheral tissues, but the presence of DCs in blood is scarce and they are difficult to obtain in sufficient numbers from tissues. In addition, one may encounter large donor variations. The use of cell lines would overcome these challenges, but may hold insufficient similarity with primary DCs with respect to their response to environmental stimuli, ability to capture antigens, mature, migrate, produce cytokines, present antigens and activate T cells. Here we summarize a series of commonly used functional DC assays below and discuss the performance of cell lines and primary cells (summarized in Table 17.2).
3.1 DC Cell Lines
Most DC cell lines were differentiated from leukemia-derived cells, originating from myelogenous or monocytic lineage: the cytokine independent cell lines THP-1, U937, KG-1, HL-60 and the cytokine dependent cell line Mutz-3 are the most commonly used (Table 17.1) (Berges et al. 2005; van Helden et al. 2008; Santegoets et al. 2008). The use of these lines has the advantage of the availability of large numbers of synchronized cells with a long life span, but the association with selective DC subsets in peripheral blood and mucosal surfaces is largely unclear. All of these cells are leukemic, representing DC precursor stages and may often need the addition of specific growth and differentiation factors to induce at least some characteristics of differentiated DCs. MUTZ-3 is suggested to have the highest DC differentiation capacity (Santegoets et al. 2008) and may therefore be most representative for in vivo DCs.
3.2 Isolating Primary DCs from Blood
DCs can be directly isolated from PB using their unique surface markers (Fig. 17.1) and utilizing either magnetic bead separation or flow cytometric cell sorting (Vremec and Shortman 1997; Dzionek et al. 2000; Shortman and Liu 2002). Since the percentage of DCs in PB is generally low (cDC: 0.6 % and pDC 0.2 % of all PBMC), DC-enrichment (by depletion of cells expressing lineage markers CD3, CD14, CD16, CD19, CD20, and CD56) is often used. For magnetic bead sorting, separation/isolation kits are commercially available from companies such as Miltenyi Biotec and StemCell Technologies. Preferably, freshly isolated PBMCs obtained by a density gradient of Ficoll-Hypaque, are suspended in cell isolation buffer as per manufacturer’s instructions. To optimize survival of primary DCs after isolation it may be helpful to add survival factors to the culture medium: 800 U/ml GM-CSF for cDCs, like CD1c or CD141 DCs, or 10 ng/ml IL-3 for pDCs.
Culturing peripheral blood-derived CD1c (BDCA1) DCs for 2 days with GM-CSF, vitamin D and Retinoic Acid (RA) induced high expression of RALDH as well as the ability to induce gut-homing receptors resembling the gut-resident CD103+Sirpα+ (DP) DCs to some extent (Sato et al. 2013).
Although using primary DCs directly isolated from the blood is feasible for most academic research labs and will probably resemble the in vivo DC biology most closely, the limited amount of primary DCs in PB is an obvious obstacle when many conditions and functional effects need to be assessed.
3.3 CD34+-Derived DCs
Another alternative is to generate DCs from myeloid precursors. CD34 is a marker expressed on haematopoietic stem cells and early myeloid progenitors that contain high proliferative potential. The CD34+ cells can therefore first be expanded followed by a differentiation towards DCs. CD34+ cells can be isolated from bone marrow (BM), peripheral blood (PB) or from umbilical cord blood (CB) using magnetic labelling (Kato and Radbruch 1993).
Firstly, CD34+ cells can be expanded using different combinations of cytokines and growth factors. Flt-3L, TPO, SCF, IL-3, and IL-6 are early-acting cytokines that support the proliferation of DC precursors from CD34+ HPC. Two different cocktails, Flt3-L, TPO, SCF and Flt3-L, SCF, IL-3, IL-6 (FS36), were compared for their capacity to induce proliferation of CD34+ HPC (Bontkes 2002). FS36 showed the greatest ability to expand the CD34+ HPCs. Expanded CD34+ cells can be differentiated towards different DC subtypes i.e. BDCA3+ DCs up to only 3 % (Poulin et al. 2010), or pDCs up to 5 % (Demoulin et al. 2012). Addition of TGF-beta during differentiation enhances the formation of langerin+ LC (Soulas et al. 2006; Caux et al. 1992; Szabolcs et al. 1995).
Established protocols to culture LCs, dermal DCs, BDCA3+ DCs and pDCs can be found in literature and are summarized below (Fig. 17.2).
3.4 Monocyte-Derived DCs
Monocytes can be selected via adherence or CD14+ isolation with magnetic beads. For adherence, PBMCs are resuspended in culture medium and placed in a humidified incubator maintained at 37 °C and 5 % CO2, to allow the monocyte precursors to adhere. After 1 h, non-adherent cells are simply removed by firmly tapping the flask and isolating non-adherent cells. For bead selection, PBMCs are resuspended in cell isolation buffer as per manufacturer’s instructions, followed by isolation using a commercially available kit. The isolated monocytes can be frozen for later use.
Once the monocytes are selected, they can be differentiated towards DCs with culture-medium (e.g. X-vivo) supplemented with 800–1,000 U/ml GM-CSF and 300–500 U/ml IL-4 (37 °C and 5 % CO2) for 7 days. After the 6/7-day culture period, the immature DCs can be used for testing the immune modulatory effects of food bioactives.
To generate mucosal CD103+Sirpα+ (DP) resembling moDCs, RA, the even more potent RARalpha agonist AM580 or TGFbeta, can be added to the culture (Hartog et al. 2013; Martin et al. 2014). RA-induced moDCs express CD103 and can induce increased percentages of FoxP3+ in allogeneic CD4+ cells. RA-induced moDC also express high levels of IL-22BP which is comparable to the CD11b+CD103+ DCs found in mouse LP and MLN (Martin et al. 2014).
4 Asses Viability
To validate the generation of DCs in these cultures, the purity of DCs can be assessed by flow cytometric analysis of the surface markers CD11c and HLA-DR. This staining can be expanded with markers, depending on the specific protocol used (for instance CD14 expression on MoDC or CD1c on CD34-derived DC.) Viability can be checked by adding a viability dye (e.g. 7AAD) to the FACS panel, or count the amount of cells with Tryphan blue with a microscope (Table 17.2).
5 Experimental Readout
Although bioactive food components have been shown to interact with many different types of cells, it remains a legitimate question whether DCs exposed to food bioactives may in turn affect features of the innate or adaptive immune system, in particular T cells. Some food bioactives may be associated with the development of allergic or autoimmune-like clinical symptoms in susceptible individuals. The clinical manifestation, ranging from mild to severe and life-threatening, vary considerably for different compounds and for the same compound in different subjects and may be idiosyncratic in nature. They comprise pseudo-allergic reactions (interference with immunological effector mechanisms and cells, such as mast cells), compound-allergic reactions (activation of compound-specific T and B cells), and compound-induced autoimmune reactions. Although the induction of allergic or autoimmune responses requires DC activation, food bioactives may also inhibit DC activation. This may represent an advantageous effect of food bioactives in reducing (chronic) inflammatory response like asthma, inflammatory bowel disease, etcetera, by reducing the DC activity and possibly inducing regulatory T cells.
Different assays can be performed to test the DC’s responsiveness to bioactives for the prediction of the subsequent immune modulatory effect of that specific compound. These assays include the monitoring of phenotypic markers expressed on the surface of DCs indicating their differentiation, activation and their potentially stimulating effect on T cells. Nevertheless, even with a clear outcome of these assays, it remains to be seen whether indeed antigen-specific T cells with the potential to induce adverse effects in vivo will be induced. In this regards it is important to consider whether the food bioactive contains potential antigens, are able to form neo-antigens or merely function as adjuvants for responses against other components in the food or play a role in sensitization to auto-antigens.
5.1 Co-stimulation
DCs may undergo a number of phenotypical changes, following maturation by the uptake and processing of an antigen. The process of DC maturation, in general, involves an increase in the surface expression of co-stimulatory molecules, like CD40, CD80, CD83, and CD86, which can be measured by flow cytometry. As a negative control unloaded DCs can be taken along, as well as a DC positively stimulated with a known maturation cocktail, like IL-1beta, IL-6, TNF and PGE-2 or TLR ligands.
5.2 Cytokine Production
Besides up-regulation of co-stimulatory molecules, DCs produce a large variety of cytokines that could be either pro/anti-inflammatory or skew the phenotype of T cells. Therefore, a quite simple procedure to test the activity of the DCs towards the food bioactives is the measurement of cytokine levels. A wide variety of cytokines may be expressed by mature DCs including IL-1 alpha, IL-1 beta, IL-4, IL-6, IL-8, IL-10, IL-12, IL-15, IL-16, IL-17, IL-18, IFN-alpha, IFN-beta, IFN-gamma, and TNF. These cytokines can be measured by ELISA, or multiplex assays.
5.3 Other DC Readouts
Co-stimulation and cytokine production are effect parameters used in the vast majority of studies on effects of food bioactives on DCs (see Table 17.3 for an overview). Studying the induction of specific T cells is complicated due to the low frequency of T cell precursors with the corresponding TCR and requires extensive culture conditions and broad read-out tools as the TCR specificity in these conditions is unknown. With regard to the T cell stimulatory potential of DCs the mixed lymphocyte reaction (MLR), in which DCs are used to stimulate alloreactive (naïve) T cells, can be considered. Proliferation in combination with cytokine production can be used as a read-out for T cell skewing.
6 In Vitro Studies on Food Bioactives Using DCs (Table 17.3)
The immunomodulatory effects of food bioactives can be broadly divided in ‘activation’, ‘suppression’ or ‘no detectable effect’. Table 17.3 shows that with regard to food proteins immune activation is generally the anticipated endpoint. It should however be considered that other food constituents instead of the protein itself, may stimulate DCs to internalize and present food proteins, and therefore may be involved in the risk for hypersensitivity. Immune suppression in DCs assays is mostly studied in combination with a trigger known to activate the DCs (TLR ligands i.e. LPS, CT or CD40 ligation). Inhibition of DC activation has been studied using probiotics, vitamins, polyphenols (Del Cornò et al. 2014a), isoflavones and curcuminoids. Inhibition of DC activation may be relevant for people suffering from chronic inflammation not only by reducing inflammation directly but also by preventing the risk for developing cancer, which is associated with chronic inflammation. Potential harmful effects of non-specific immune suppression include an increased risk of infection and also here the development of cancer. When there is no direct effect of the food bioactives on the DCs with the assays mentioned above, this does not mean that exposure to the compound is without risk. Indirect effects of the bioactives through their effects on other cells or the effects of their metabolites must be taken into consideration.
7 Critical Notes
Extensive comparative studies using different DC assays with proven immune active compounds and food bioactive compound are lacking.
With regard to some of the DC parameters used to study immune modulatory effects of food bioactives, the expression of co-inhibitory molecules (e.g. PDL1 and PDL2) has not been intensively studied. This family of molecules has however a large impact on the immune response and may therefore also be considered in future testing.
As mentioned before, the DCs are the sentinels of the immune system that gather not only by taking up or sensing the food bioactives only, but continuously receive input from cells and factors in their microenvironment. Potential responses should therefore be evaluated in the light of the local environment. For instance, the choice for a particular DC model/subset may be dependent on the availability of certain compounds/entry site, which may relate to the size, solubility and formulation of the compound of interest.
In conclusion, studying the effects of food bioactives on DC (subsets) is an important step in addressing the immune modulating potential of these compounds in vivo, but should be part of a more extended monitoring program involving studies on the compound’s availability, factors of the microenvironment, and effects of metabolites/neo-epitopes.
References
Anderson AO, Shaw S (2005) Conduit for privileged communications in the lymph node. Immunity 22:3–5. doi:10.1016/j.immuni.2005.01.003
Bain CC, Scott CL, Uronen-Hansson H et al (2012) Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C. Mucosal Immunol 6:498–510. doi:10.1038/mi.2012.89
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252. doi:10.1038/32588
Berges C, Naujokat C, Tinapp S et al (2005) A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun 333:896–907. doi:10.1016/j.bbrc.2005.05.171
Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C et al (2012) Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS One 7:e43197. doi:10.1371/journal.pone.0043197
Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C et al (2013) Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS One 8:e59370. doi:10.1371/journal.pone.0059370
Bernardo D, Sánchez B, Al-Hassi HO et al (2012) Microbiota/host crosstalk biomarkers: regulatory response of human intestinal dendritic cells exposed to Lactobacillus extracellular encrypted peptide. PLoS One 7:e36262. doi:10.1371/journal.pone.0036262
Bettelli E, Korn T, Oukka M, Kuchroo VK (2008) Induction and effector functions of T(H)17 cells. Nature 453:1051–1057. doi:10.1038/nature07036
Bhardwaj N, Bender A, Gonzalez N et al (1994) Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest 94:797–807. doi:10.1172/JCI117399
Bontkes HJ, De Gruijl TD, Schuurhuis GJ, Scheper RJ, Meijer CJ, Hooijberg E (2002) Expansion of dendritic cell precursors from human CD34(+) progenitor cells isolated from healthy donor blood; growth factor combination determines proliferation rate and functional outcome. J Leukoc Biol 72(2):321–329
Brosbøl-Ravnborg A, Bundgaard B, Höllsberg P (2013) Synergy between vitamin D3 and Toll-like receptor agonists regulates human dendritic cell response during maturation. Clin Dev Immunol 2013:1–8. doi:10.1155/2013/807971
Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J (1992) GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360:258–261. doi:10.1038/360258a0
Del Cornò M, Scazzocchio B, Masella R, Gessani S (2014a) Regulation of dendritic cell function by dietary polyphenols. Crit Rev Food Sci Nutr 140618104333000. doi:10.1080/10408398.2012.713046
Del Cornò M, Varano B, Scazzocchio B et al (2014b) Protocatechuic acid inhibits human dendritic cell functional activation: role of PPARγ up-modulation. Immunobiology 219:416–424. doi:10.1016/j.imbio.2014.01.007
Demoulin S, Roncarati P, Delvenne P, Hubert P (2012) Production of large numbers of plasmacytoid dendritic cells with functional activities from CD34(+) hematopoietic progenitor cells: use of interleukin-3. Exp Hematol 40:268–278. doi:10.1016/j.exphem.2012.01.002
den Hartog G, van Altena C, Savelkoul HFJ, van Neerven RJJ (2013) The mucosal factors retinoic acid and TGF-β1 induce phenotypically and functionally distinct dendritic cell types. Int Arch Allergy Immunol 162:225–236. doi:10.1159/000353243
Dzionek A, Fuchs A, Schmidt P et al (2000) BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 165:6037–6046
Evrard B, Coudeyras S, Dosgilbert A et al (2011) Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS One 6:e18735. doi:10.1371/journal.pone.0018735
Frankenberger M, Hofer TPJ, Marei A et al (2012) Transcript profiling of CD16-positive monocytes reveals a unique molecular fingerprint. Eur J Immunol 42:957–974. doi:10.1002/eji.201141907
Giordani L, Del Pinto T, Vincentini O et al (2014) Two wheat decapeptides prevent gliadin-dependent maturation of human dendritic cells. Exp Cell Res 321:248–254. doi:10.1016/j.yexcr.2013.11.008
Gretz JE, Anderson AO, Shaw S (1997) Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev 156:11–24
Hart AL, Lammers K, Brigidi P et al (2004) Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 53:1602–1609. doi:10.1136/gut.2003.037325
Hu ZB, Ma W, Zaborski M et al (1996) Establishment and characterization of two novel cytokine-responsive acute myeloid and monocytic leukemia cell lines, MUTZ-2 and MUTZ-3. Leukemia 10:1025–1040
Kapsenberg ML (2003) Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3:984–993. doi:10.1038/nri1246
Katayama S, Kukita T, Ishikawa E et al (2013) Food chemistry. Food Chem 138:757–761. doi:10.1016/j.foodchem.2012.10.076
Kato K, Radbruch A (1993) Isolation and characterization of CD34+ hematopoietic stem cells from human peripheral blood by high-gradient magnetic cell sorting. Cytometry 14:384–392. doi:10.1002/cyto.990140407
Koeffler HP, Bar-Eli M, Territo MC (1981) Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res 41:919–926
Koski GK, Schwartz GN, Weng DE et al (1999) Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood 94:1359–1371
Langerhans P (1868) Ueber die Nerven der menschlichen Haut. Virch Arch Pathol Anat 44:325–337
Lindstedt M, Lundberg K, Borrebaeck CAK (2005) Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J Immunol 175:4839–4846
Mann ER, Landy JD, Bernardo D et al (2013) Intestinal dendritic cells: their role in intestinal inflammation, manipulation by the gut microbiota and differences between mice and men. Immunol Lett 150:30–40. doi:10.1016/j.imlet.2013.01.007
Martin JCJ, Bériou G, Heslan M et al (2014) Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol 7:101–113. doi:10.1038/mi.2013.28
Masilamani M, Wei J, Bhatt S, et al (2011) Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J Allergy Clin Immunol 128:1242–1250.e1. doi:10.1016/j.jaci.2011.05.009
McLellan AD, Sorg RV, Williams LA, Hart DN (1996) Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand-dependent pathway. Eur J Immunol 26:1204–1210. doi:10.1002/eji.1830260603
Palova-Jelinkova L, Rozkova D, Pecharova B et al (2005) Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J Immunol 175:7038–7045. doi:10.4049/jimmunol.175.10.7038
Piemonti L, Monti P, Sironi M et al (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164:4443–4451
Poulin LF, Salio M, Griessinger E et al (2010) Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8 + dendritic cells. J Exp Med 207:1261–1271. doi:10.1084/jem.20092618
Rivollier A, He J, Kole A et al (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209:139–155. doi:10.1084/jem.20101387
Robbins SH, Walzer T, Dembélé D et al (2008) Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol 9:R17. doi:10.1186/gb-2008-9-1-r17
Rogers NM, Kireta S, Coates PTH (2010) Curcumin induces maturation-arrested dendritic cells that expand regulatory T cells in vitro and in vivo. Clin Exp Immunol 162:460–473. doi:10.1111/j.1365-2249.2010.04232.x
Roozendaal R, Mempel TR, Pitcher LA et al (2009) Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30:264–276. doi:10.1016/j.immuni.2008.12.014
Sakaguchi S, Ono M, Setoguchi R et al (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212:8–27. doi:10.1111/j.0105-2896.2006.00427.x
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118
Santegoets SJAM, van den Eertwegh AJM, van de Loosdrecht AA et al (2008) Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol 84:1364–1373. doi:10.1189/jlb.0208092
Sato T, Kitawaki T, Fujita H et al (2013) Human CD1c+ myeloid dendritic cells acquire a high level of retinoic acid-producing capacity in response to vitamin D3. J Immunol 191:3152–3160. doi:10.4049/jimmunol.1203517
Segura E, Touzot M, Bohineust A et al (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38:336–348. doi:10.1016/j.immuni.2012.10.018
Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS (2008) Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun 374:431–436. doi:10.1016/j.bbrc.2008.07.051
Shortman K, Liu Y-J (2002) Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–161. doi:10.1038/nri746
Shreffler WG, Castro RR, Kucuk ZY et al (2006) The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol 177:3677–3685
Sixt M, Kanazawa N, Selg M et al (2005) The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22:19–29. doi:10.1016/j.immuni.2004.11.013
Smole U, Wagner S, Balazs N et al (2010) Bet v 1 and its homologous food allergen Api g 1 stimulate dendritic cells from birch pollen-allergic individuals to induce different Th-cell polarization. Allergy 65:1388–1396. doi:10.1111/j.1398-9995.2010.02407.x
Soulas C, Arrighi J-F, Saeland S et al (2006) Human CD34+CD11b− cord blood stem cells generate in vitro a CD34−CD11b+ subset that is enriched in langerin+ Langerhans dendritic cell precursors. Exp Hematol 34:1471–1479. doi:10.1016/j.exphem.2006.06.011
Steinman RM, Cohn ZA (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137:1142–1162
Sugimura T, Jounai K, Ohshio K et al (2013) Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin Immunol 149:509–518. doi:10.1016/j.clim.2013.10.007
Szabolcs P, Moore MAS, Young JW (1995) Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol 154:5851–5861
Tamoutounour S, Henri S, Lelouard H et al (2012) CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42:3150–3166. doi:10.1002/eji.201242847
Tsuchiya S, Yamabe M, Yamaguchi Y et al (1980) Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176
van Helden SFG, van Leeuwen FN, Figdor CG (2008) Human and murine model cell lines for dendritic cell biology evaluated. Immunol Lett 117:191–197. doi:10.1016/j.imlet.2008.02.003
Vremec D, Shortman K (1997) Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol 159:565–573
Watchmaker PB, Lahl K, Lee M et al (2014) Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol 15:98–108. doi:10.1038/ni.2768
Wei J, Bhatt S, Chang LM et al (2012) Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS One 7:e47979. doi:10.1371/journal.pone.0047979
Ziegler-Heitbrock L, Ancuta P, Crowe S et al (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80. doi:10.1182/blood-2010-02-258558
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Plantinga, M., de Haar, C., Nierkens, S. (2015). Dendritic Cells. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)