Abstract
IPEC-J2 cells are intestinal porcine enterocytes isolated from the jejunum of a neonatal unsuckled piglet. The IPEC-J2 cell line is unique as it is derived from the small intestine and is neither transformed nor tumorigenic in nature. IPEC-J2 cells mimic the human physiology more closely than any other cell line of non-human origin. Therefore, it is an ideal tool to study epithelial transport, interactions with enteric bacteria, effects of probiotic microorganisms and the effect of nutrients and other feedstuffs on a variety of widely used parameters (e.g. transepithelial electrical resistance (TEER), permeability, metabolic activity) reflecting epithelial functionality.
IPEC-J2 cells undergo in culture a process of spontaneous differentiation that leads to the formation of a polarized monolayer with low or high TEER, depending on the type of serum added to the culture medium, within 1–2 weeks. Porcine serum gives rise to low resistance and normal active transport rates, enabling comparison with the in vivo situation. The high resistance caused by fetal bovine serum can be beneficial to use when investigating compounds having a negative effect on the monolayer permeability or tight junction structures.
There are still many opportunities for exploring the use of these cells as the available research is limited. This chapter will cover the origin, characteristics and methods of the use of IPEC-J2 cells as an in vitro model for several research applications, as well as comparisons between IPEC-J2 cells and other epithelial cell lines.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- IPEC-J2
- Cell line
- Small intestinal epithelium
- Porcine origin
- Transepithelial electrical resistance
- Non-transformed
- Continuous
1 Origin
The intestinal porcine enterocyte cell line (IPEC-J2) is a non-transformed, permanent intestinal cell line (Fig. 12.1). These secondary cells were originally isolated from the jejunal epithelium of a neonatal unsuckled piglet in 1989 by Helen Berschneider at the University of North Carolina (Berschneider 1989). Given the correct culture conditions, these cells will divide and grow for an infinite number of passages in vitro. To date, they have been cultured continuously for up to 98 passages. IPEC-J2 cells were first used to investigate transepithelial ion transport and enterocyte differentiation (Berschneider 1989). They have already been shown to be a valuable tool in the characterization of epithelial cell interactions with enteric bacteria and viruses providing insight into initial host-pathogen and non-pathogen (e.g. commensal or probiotic) interactions (Brosnahan and Brown 2012).
2 Special Features/Morphology/Receptors
The strength of the IPEC-J2 cell line as an in vitro model originates from its morphological and functional similarities with intestinal epithelial cells in vivo. IPEC-J2 cells have microvilli on their apical side and tight junctions sealing neighboring cells together (Schierack et al. 2006). To date, no brush border enzyme activity has been investigated in IPEC-J2 cells. IPEC-J2 cells form polarized monolayers when cultured on 0.4 μm pore-size filters, with or without a collagen basis. High transepithelial electrical resistance (TEER) values and low active transport rates are obtained when culturing the IPEC-J2 cells in fetal bovine serum (FBS) (Geens and Niewold 2011). These atypically high TEER values can be beneficial to use when investigating compounds having a negative effect on the monolayer permeability or tight junction structures (Vergauwen et al. 2015). Porcine serum (PS) resulted in significantly lower TEER values and higher active transport rates comparable to the in vivo situation (Zakrzewski et al. 2013). When IPEC-J2 cells are grown in 10 % PS they will become taller and smaller in diameter, increasing the tight junction ultrastructure (Zakrzewski et al. 2013).
The protein expression of claudin-1, -3, -4, -5, -7, -8, -12, tricellulin, occludin, E-cadherin and zonula occludens-1 (ZO-1) by IPEC-J2 cells has been confirmed by immunoblotting (Zakrzewski et al. 2013). On the other hand, IPEC-J2 cells do not express claudin-2 and -15 resulting in lower cation selectivity, augmenting the ion permeability of tight junctions compared to the porcine jejunum (Zakrzewski et al. 2013; Schierack et al. 2006).
IPEC-J2 cells express and produce cytokines, defensins, toll-like receptors and mucins. The presence of glycocalyx-bound mucins like Muc1 and Muc3 has been confirmed in IPEC-J2 cells, respectively by RT-PCR and ELISA (Schierack et al. 2006). The expression of the Muc2, the major gel-forming mucin in the human small intestine, was not detectable by RT-PCR (Schierack et al. 2006). Thus, the mucus production by IPEC-J2 cells is not comparable to the in vivo situation. The mucus layer is only a very thin layer that is superimposed on the IPEC-J2 cells when cultured with fetal bovine serum. However, PAS staining showed an increase in mucus production when IPEC-J2 cells were cultured with porcine serum (Zakrzewski et al. 2013).
IPEC-J2 cells express proteins such as MHC I and secrete cytokines like GM-CSF and TNF-α that can establish communication between enterocytes and the immune system. Furthermore, mRNA expression of TLR-1, -2, -3, -4, -5, -6, -8, -9, -10 and IL-1α, -1β, -6, -7, -8, -12A, -12B, -18 has been confirmed in IPEC-J2 cells. TLR-2, -3, -5, -9 and IL-6, -8 proteins were also detected in these cells (Brosnahan and Brown 2012; Arce et al. 2010; Burkey et al. 2009).
IPEC-J2 cells express the F4 fimbrial receptor but not the F18 fimbrial receptor as this has been correlated to older pigs (3–23 weeks). These features make IPEC-J2 cells an interesting in vitro model to investigate the pathogenesis of zoonotic enteric infections that also affect humans.
3 Stability/Consistency/Reproducibility of the System
In current research IPEC-J2 cells have been used until passage 98. However, most studies do not mention the passage number. Interlaboratory result evaluation presented a consensus that a confluent IPEC-J2 cell monolayer grown in 5 % fetal bovine serum corresponds to a TEER value of 1,000 Ω × cm2. TEER experiments are usually started at 1,000–3,000 Ω × cm2. These values are reached after 4–9 days of culturing on an insert with a semi-permeable membrane (0.4 μm pore size) with or without collagen coating. However, when IPEC-J2 cells are incubated with 10 % PS instead of 5 % FBS TEER values of 400–500 Ω × cm2 are obtained. These TEER values are comparable to those found in Caco-2 cell cultures and in vivo.
The amount of cells seeded on the insert depends on the insert size and brand. It has been demonstrated that seeding density can influence TEER values over time, as well as proliferation capacity and functionality. When a 12-well plate insert is seeded with 1–10 × 105 cells/insert, minimal cell division is needed.
Results obtained from microbial investigations using IPEC-J2 cells show strong correlation with mucosal explants and in vivo. However, various EHEC mutants adhere differently to IPEC-J2 cells and porcine ileal loops. This indicates that different model systems may behave differently (Yin et al. 2009). Nonetheless, the ability of some EHEC mutants to adhere to IPEC-J2 cells and ileal loops was highly correlated.
4 Relevance to Human In Vivo Situation
IPEC-J2, IPEC-1 and IPI-2I are three widely used porcine small intestinal cell lines (Arce et al. 2010). IPI-2I cells have been transformed with an SV40 plasmid, whereas IPEC-J2 and IPEC-1 are non-transformed cell lines. IPI-2I cells were isolated from the ileum, IPEC-J2 cells from the jejunum and IPEC-1 cells were isolated from a mixture of ileal and jejunal tissue. IPEC-1 and IPEC-J2 cells were both isolated from one day old piglets, whereas IPI-2I cells were isolated from an adult boar (Berschneider 1989). Furthermore, IPEC-J2 cells, unlike IPI-2I cells, form a polarized monolayer, promoting S. typhimurium invasion and replication (Boyen et al. 2009).
Several normal and transformed cell lines are used in food science (Cencic and Langerholc 2010). Caco-2, T84, HT-29, HUTU-80 and SW620 are the most widely used human intestinal cell lines. The majority of human intestinal cell lines is isolated from the colon, and most are tumorigenic. The HUTU-80 cell line is the only widely available small intestinal human cell line. It was derived from the duodenum, but is also cancerous (Brosnahan and Brown 2012).
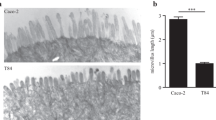
A drug transport permeability study showed that IPEC-J2 cells form a tighter monolayer compared to Caco-2 cells (Pisal et al. 2008). On the other hand, Caco-2 and T84 cells produce a more pronounced mucus layer compared to IPEC-J2 cells (Navabi et al. 2013). HT29, T84, Caco-2, and SW620 are all negative for IL-2, IL-4, and IFN-γ, while mRNA for IL-1α, IL-8, and TNF-α is variable amongst the human cell lines, but is present in IPEC-J2 cells (Eckmann et al. 1993). Existing cancer-derived cell lines can have limitations such as an altered glycosylation pattern and an aberrant protein expression that define the epithelial character as well as unresponsiveness to hormones or cytokines (Peracaula et al. 2008).
Two major advantages favor IPEC-J2 cells as a model of normal intestinal epithelial cells compared to transformed cell lines: (1) as a permanent cell line they maintain their differentiated characteristics and exhibit strong similarities to primary intestinal epithelial cells, and (2) IPEC-J2 cells have the advantage of being directly comparable to the experimental animal that is used as an in vivo model for humans. Of all non-primates, the gastrointestinal (GI) tract of pigs is the most appropriate in vivo model as it is similar in size, weight, anatomy and physiology as the human GI system (Deglaire and Moughan 2012).
Due to the close similarity between swine and human intestinal function, studies with IPEC-J2 cells provide valuable insights into the pathogenesis of zoonotic enteric infections that also affect humans (Skjolaas et al. 2006). Extrapolating information can be difficult as in vitro, ex vivo and in vivo cells or tissue can respond differently to environmental stimuli (e.g. diet). Indeed, diet-induced gene expression patterns differ between IPEC-J2 cells and intestinal tissue from preterm and newborn piglets, making interpretation rather difficult (Støy et al. 2013). However, an in vitro model is still a valuable tool to investigate a limited number of factors in a standardized, regulated setting. A comparison of normal diploid IPEC-J2 cells with other transformed or tumorigenic cell lines can give more insight when investigating gene expression and to greater extent the effects of bioactives on intestinal health.
5 General Protocol
5.1 Culture Conditions
The IPEC-J2 cells are cultured in DMEM/F-12 mix (Dulbecco’s Modified Eagle Medium, Ham’s F-12 mixture) and supplemented with HEPES, fetal bovine serum (FBS) or porcine serum (PS), insulin/transferrin/selenium (ITS), penicillin/streptomycin and cultivated in a humid environment at 37 °C with 5 % CO2. The IPEC-J2 cells are usually grown for 1–2 weeks before initiating an experiment. When studying TEER and permeability, IPEC-J2 cells are commonly seeded (1 × 105 cells/well) at confluence in a ‘Boyden chamber’ insert (upper chamber, apical) on a polyethylene terephthalate (PET) membrane (1.12 cm2, pore size 0.4 μm) in a 12-well plate (lower chamber, basolateral). Cells are seeded (1 × 105/well) in a 12-well plate (flat bottom) to investigate the intracellular oxidative stress and wound healing capacity. Cells are seeded (0.5 × 104 cells/well) in a 96-well plate (flat bottom) to assess viability. The IPEC-J2 cell line is an easy to use and robust cell line, exhibiting structural and functional differentiation pattern characteristics of mature enterocytes (Geens and Niewold 2011).
5.2 Experimental Readout
An increasing number of studies use IPEC-J2 cells to investigate interactions of various animal and human pathogens, including Salmonella enterica and pathogenic Escherichia coli (Boyen et al. 2009; Veldhuizen et al. 2009). Pathogenic permeation (e.g. E. coli) is usually presented as colony forming units (CFU). IPEC-J2 cells have also been employed as an initial screening tool for adhesiveness and anti-inflammatory properties of potential probiotic microorganisms. IPEC-J2 cells were also used to investigate the effect of prebiotics on the adhesion of probiotic bacterial strains to these cells. Addition of 200 mM calcium has been shown to increase adhesion (Marcinakova et al. 2010), while magnesium and zinc ions had no influence (Larsen et al. 2007). Innate immune responses (e.g. increase in porcine β-defensin 1 and 2 gene expression) in relation to environmental stimuli (e.g. diet or infection) are investigated with relevance for human and porcine intestinal diseases, specifically in newborns (Schierack et al. 2006; Burkey et al. 2009; Veldhuizen et al. 2009). Numerous studies used IPEC-J2 cells to investigate feedstuffs and antioxidants in relation to inflammation, intestinal permeability and wound healing capacity (Hermes et al. 2011; Ma et al. 2012; Pan et al. 2013; Vergauwen et al. 2015).
Permeability is expressed using TEER, either presented as absolute values or as percentages of control or time point (TEERtx/TEERt0). The net value of the TEER (Ω × cm2) needs to be corrected for background resistance by subtracting the contribution of the cell-free filter and the medium (80–150 Ω × cm2). Alternatively fluorescein isothiocyanate (FITC)-dextran of 4 kDa (FD-4) permeability can be used to indicate monolayer integrity. FD-4 permeability results are presented as a percentage of control or as absolute quantities or concentrations (e.g. picomoles).
Viability and cytotoxicity are most commonly analyzed using the neutral red method, lactate dehydrogenase release or the MTT reduction assay and presented as percentages of control or absorbance values (Table 12.1).
5.3 Sample Preparation
It is important only to incubate sterile filtered samples on the IPEC-J2 cells. Otherwise, interpretation of the results will be ambiguous as the effect cannot be contributed to an impurity, a pathogen or the agent of interest.
Samples that are not readily dissolved in water can be dissolved in ethanol or DMSO. The effect of different concentrations of ethanol or DMSO for either 1 or 18 h on the viability of IPEC-J2 cells was investigated (Fig. 12.2). Results show that short and long term incubation do not favor DMSO or ethanol at concentrations below 1 %. DMSO is favored for long-term incubation at concentrations above 1 %. However, concentrations exceeding 1 % are not recommended. Furthermore, it is always important to minimize the concentration of DMSO or ethanol when solubilizing a compound.
6 Conclusion
In summary, the IPEC-J2 cell model provides a perfect tool to investigate intestinal epithelial function. This porcine cell line is closely related to the human in vivo situation and is not cancerous compared to other human (small) intestinal cell lines. The tumorigenic nature of the human intestinal cell lines can influence gene expression, and transformed cell lines are usually more resistant to stress or cytotoxic insults. This will result in ambiguous information and an underestimation of cytotoxic compounds. Furthermore, IPEC-J2 cell monolayers are ready for experimentation after 1–2 weeks which is a lot faster compared to the 21-day culturing time of Caco-2 cells. In conclusion, IPEC-J2 cells are an ideal small intestinal enterocyte model to study effects of food bioactives in the gut prior to in vivo evaluation.
References
Arce C, Ramirez-Boo M, Lucena C, Garrido JJ (2010) Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp Immunol Microbiol Infect Dis 33(2):161–174. doi:10.1016/j.cimid.2008.08.003
Awad WA, Aschenbach JR, Zentek J (2012) Cytotoxicity and metabolic stress induced by deoxynivalenol in the porcine intestinal IPEC-J2 cell line. J Anim Physiol Anim Nutr 96(4):709–716. doi:10.1111/j.1439-0396.2011.01199.x
Berschneider HM (1989) Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology 96:A41
Bondzio A, Lodemann U, Weise C, Einspanier R (2013) Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression. PLoS One 8(7):e67079. doi:10.1371/journal.pone.0067079
Boyen F, Pasmans F, Van Immerseel F, Donne E, Morgan E, Ducatelle R, Haesebrouck F (2009) Porcine in vitro and in vivo models to assess the virulence of Salmonella enterica serovar Typhimurium for pigs. Lab Anim 43(1):46–52. doi:10.1258/la.2007.007084
Brosnahan AJ, Brown DR (2012) Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol 156(3–4):229–237. doi:10.1016/j.vetmic.2011.10.017
Burkey TE, Skjolaas KA, Dritz SS, Minton JE (2009) Expression of porcine Toll-like receptor 2, 4 and 9 gene transcripts in the presence of lipopolysaccharide and Salmonella enterica serovars Typhimurium and Choleraesuis. Vet Immunol Immunopathol 130(1–2):96–101. doi:10.1016/j.vetimm.2008.12.027
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L, Zhu S, Xu J (2013) Pre-protective effect of lipoic acid on injury induced by H(2)O (2) in IPEC-J2 cells. Mol Cell Biochem. doi:10.1007/s11010-013-1595-9
Cencic A, Langerholc T (2010) Functional cell models of the gut and their applications in food microbiology—a review. Int J Food Microbiol 141(Suppl 1):S4–S14. doi:10.1016/j.ijfoodmicro.2010.03.026
Deglaire A, Moughan PJ (2012) Animal models for determining amino acid digestibility in humans – a review. Br J Nutr 108(Suppl 2):S273–S281. doi:10.1017/s0007114512002346
Eckmann L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF (1993) Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 105(6):1689–1697
Geens MM, Niewold TA (2011) Optimizing culture conditions of a porcine epithelial cell line IPEC-J2 through a histological and physiological characterization. Cytotechnology 63(4):415–423. doi:10.1007/s10616-011-9362-9
Hermes RG, Manzanilla EG, Martin-Orue SM, Perez JF, Klasing KC (2011) Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88). Comp Immunol Microbiol Infect Dis 34(6):479–488. doi:10.1016/j.cimid.2011.08.006
Larsen N, Nissen P, Willats WG (2007) The effect of calcium ions on adhesion and competitive exclusion of Lactobacillus ssp. and E. coli O138. Int J Food Microbiol 114(1):113–119. doi:10.1016/j.ijfoodmicro.2006.10.033
Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF (2012) Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci 90(Suppl 4):266–268. doi:10.2527/jas.50965
Marcinakova M, Klingberg TD, Laukova A, Budde BB (2010) The effect of pH, bile and calcium on the adhesion ability of probiotic enterococci of animal origin to the porcine jejunal epithelial cell line IPEC-J2. Anaerobe 16(2):120–124. doi:10.1016/j.anaerobe.2009.05.001
Navabi N, McGuckin MA, Linden SK (2013) Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS One 8(7):e68761. doi:10.1371/journal.pone.0068761
Pan L, Qin G, Zhao Y, Wang J, Liu F, Che D (2013) Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets. Int J Mol Sci 14(11):21689–21704. doi:10.3390/ijms141121689
Paszti-Gere E, Matis G, Farkas O, Kulcsar A, Palocz O, Csiko G, Neogrady Z, Galfi P (2014) The effects of intestinal LPS exposure on inflammatory responses in a porcine enterohepatic co-culture system. Inflammation 37(1):247–260. doi:10.1007/s10753-013-9735-7
Peracaula R, Barrabes S, Sarrats A, Rudd PM, de Llorens R (2008) Altered glycosylation in tumours focused to cancer diagnosis. Dis Markers 25(4–5):207–218
Pisal DS, Yellepeddi VK, Kumar A, Palakurthi S (2008) Transport of surface engineered polyamidoamine (PAMAM) dendrimers across IPEC-J2 cell monolayers. Drug Deliv 15(8):515–522. doi:10.1080/10717540802321826
Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH (2006) Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol 125(3):293–305. doi:10.1007/s00418-005-0067-z
Sewekow E, Bimczok D, Kahne T, Faber-Zuschratter H, Kessler LC, Seidel-Morgenstern A, Rothkotter HJ (2012) The major soyabean allergen P34 resists proteolysis in vitro and is transported through intestinal epithelial cells by a caveolae-mediated mechanism. Br J Nutr 1–9. doi:10.1017/s0007114511007045
Skjolaas KA, Burkey TE, Dritz SS, Minton JE (2006) Effects of Salmonella enterica serovars Typhimurium (ST) and Choleraesuis (SC) on chemokine and cytokine expression in swine ileum and jejunal epithelial cells. Vet Immunol Immunopathol 111(3–4):199–209. doi:10.1016/j.vetimm.2006.01.002
Støy ACF, Heegaard PMH, Sangild PT, Østergaard MV, Skovgaard K (2013) Gene expression analysis of the IPEC-J2 cell line: a simple model for the inflammation-sensitive preterm intestine. ISRN Genomics Volume 2013 (Article ID 980651):7 pages
Veldhuizen EJ, Koomen I, Ultee T, van Dijk A, Haagsman HP (2009) Salmonella serovar specific upregulation of porcine defensins 1 and 2 in a jejunal epithelial cell line. Vet Microbiol 136(1–2):69–75. doi:10.1016/j.vetmic.2008.09.072
Vergauwen H, Tambuyzer B, Jennes K, Degroote J, Wang W, De Smet S, Michiels J, Van Ginneken C (2015) Trolox and ascorbic acid reduce direct and indirect oxidative stress in the IPEC-J2 cells, an in vitro model for the porcine gastrointestinal tract. PLoS One 10(3):e0120485. doi:10.1371/journal.pone.0120485
Yin X, Chambers JR, Wheatcroft R, Johnson RP, Zhu J, Liu B, Gyles CL (2009) Adherence of Escherichia coli O157:H7 mutants in vitro and in ligated pig intestines. Appl Environ Microbiol 75(15):4975–4983. doi:10.1128/aem.00297-09
Zakrzewski SS, Richter JF, Krug SM, Jebautzke B, Lee IF, Rieger J, Sachtleben M, Bondzio A, Schulzke JD, Fromm M, Gunzel D (2013) Improved cell line IPEC-J2, characterized as a model for porcine jejunal epithelium. PLoS One 8(11):e79643. doi:10.1371/journal.pone.0079643
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Vergauwen, H. (2015). The IPEC-J2 Cell Line. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_12
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)