Abstract
The human colon adenocarcinoma cell line HT29, is not only used to study the biology of human colon cancers, but it is receiving special interest in studies focused on food digestion and bioavailability due to the ability to express characteristics of mature intestinal cells. In the differentiated phenotype, they are able to form a monolayer with tight junctions between cells and a typical apical brush border. In addition, these differentiated cells express brush-border-associated hydrolases typical of the small intestine although the enzymatic activity is lower than that found in vivo. Although they represent a valuable model due to their similarities with enterocytes of the small intestine, their limitations and the relevance to the in vivo situation are also considered in this chapter. The application of this cell line to transport studies of drugs and food compounds is illustrated, especially when the effect of the mucus layer is considered or used as co-culture in combination with Caco-2 cells. They have also been frequently used to study the intestinal immune response to bacterial infection, and microorganism survival, adhesion or invasion. Finally, the use of these cells to evaluate the effect of several food compounds and mucin secretion is summarized.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Origin
The human colon adenocarcinoma cell line HT29 was isolated from a primary tumor of a 44 years old Caucasian female in 1964 by Fogh and Trempe (1975). Since then, many cell lines have been derived from human colon cancers. Initially, this cell line was used to study different aspects of the biology of human cancers. However, these cells have attracted attention due to the fact they were able to express characteristics of mature intestinal cells, such as enterocytes or mucus producing cells.
2 Features and Mechanisms
These cells have shown a high rate of glucose consumption and therefore, they require high glucose concentration in the medium. Under these standard growth conditions, i.e., in the presence of 25 mM glucose and 10 % serum, these cells display an undifferentiated phenotype; they grow as a multilayer of unpolarised undifferentiated cells, and functionally they do not express any typical characteristics of intestinal epithelial cells, and they express low amounts of hydrolases. Since the discovery that the replacement of glucose by galactose in the culture medium induced a reversible enterocytic differentiation (Pinto et al. 1982), HT29 cells have become a unique model for studying the molecular mechanisms of intestinal cell differentiation. When these cells are grown under appropriate culture conditions or after treatment with different inducers, like butyrate (Augeron and Laboisse 1984) or acid (Fitzgerald et al. 1997), they can be modulated to express different pathways of enterocyte differentiation. For this reason, HT29 is considered a pluripotent intestinal cell line which can be used for the study of the structural and molecular events involved in cell differentiation.
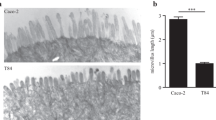
The polarised phenotype is characterised morphologically, physiologically and by biochemical markers. The differentiation is growth-related, starting after confluence (after 15 days of growth), and the cells form a monolayer with tight junctions between cells and a typical apical brush border. The brush border of these differentiated cells contains proteins which are normally present in the intestinal microvilli, such as villin. In addition, these differentiated cells express brush border-associated hydrolases typical for the small intestine, have brush border microvilli although the enzyme activities is much lower than that of the normal intestine, and do not express lactase. The maximum enzyme activities, such as sucrose-isomaltase, aminopeptidase N, dipeptidylpeptidase-IV and alkaline phosphatase, are reached after 30 days in culture. Under these conditions, p.e. growth in glucose-free medium, cell differentiation is very similar to that observed in Caco-2 cells but in HT29 the time course of the differentiation process is longer (30 days vs. 15–20 days in Caco-2); the levels of enzymatic activities are lower than in Caco-2; lactase is absent; and only 40–50 % of HT29 cells express sucrose-isomaltase (Zweibaum 1986; Zweibaum et al. 2011). However, one of the main differences between this cell line and Caco-2 is that HT29 cells can produce mucin at a relatively high level (Huet et al. 1987; Maoret et al. 1989). Stepwise adaptation of exponentially growing HT29 cells to increasing concentrations of methotrexate (MTX) (up to 10−5 mol) resulted in their transformation into mucus-secreting differentiated cells (Lesuffleur et al. 1990). As occurs under other metabolic stress conditions like glucose-deprivation, after a high rate of mortality, the resistance to MTX is associated with the cells possessing this stable differentiated mucus-secreting phenotype. Interestingly, cells adapted to low-dose MTX consist of a double population of columnar absorptive and mucus-secreting cells and, at a higher dose, cells are almost exclusively of mucus-secreting phenotype. This mucus-secreting phenotype has been used in the transport studies of different compounds, to study the mucus-inducing properties of different food compounds or in studies regarding microorganisms adhesion and survival (see Sect. 11.6).
The factors secreted from cells in culture medium include metabolites, cytokines, growth factors, etc., which are known to promote cell survival. Recently, the soluble factors secreted by HT29 and other tumor cells in basal conditions and after gamma radiation have been reported. HT29 secreted pro-inflammatory cytokines, such as, tumor necrosis factor (TNF) α and interleukins (IL) 1β and IL 6; growth factors such as platelet-derived growth factor AA and transforming growth factors (TGF) α and β; chemokines such as fractalkine, IL-8, monocyte chemoattractant protein-1 and interferon-γ-induced protein 10; pro-angiogenic factors such as IL-15 and vascular endothelial growth factor; and immune-modulatory cytokines such as granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor and IL-3. It was suggested, based on previous reports on biopsy samples, that in vivo, a similar cytokine secretion profile can be observed (Desai et al. 2013).
3 Stability, Consistency and Reproducibility
Many tumor-derived cells have been reported to show lack of culture stability and reproducibility based on the existence of irregular growth and non-specific genetic alterations (Lipps et al. 2013). To minimize these handicaps, the establishment of large cell banks is recommended to allow disposing cells at early and similar passages. On the contrary, the consistency of the assumed cell properties should be verified for highly different passages. For instance, Chen et al. (1987) proved that karyotypes were comparable for HT29 cells along more than 100 passages, which represents valuable data in the assessment of the cell line evolution. Likewise, derived cells adapted by specific treatments must be evaluated under different culture conditions as time and presence/absence of responsible agent(s). Lesuffleur et al. (1990) tested the irreversibility of the differentiation of MTX adapted cells by the comparison of the growth curve for several passages. No significant differences were observed for both 10−7 and 10−6 M MTX-derived cell lines between cells cultured with drug-enriched media and drug-free media. The stability of other differentiation characteristics was also confirmed for MTX-free cells compared with equivalent MTX-treated cells as well as themselves along several passages before.
4 Relevance to Human In Vivo Situation
As in other cancer-derived cell line models, significant differences in the gene expression of transporters and metabolic enzymes from the normal human intestinal cells can affect the suitability of the model in reflecting the in vivo permeability (Langerholc et al. 2011). Similar protein expression was found in HT29 cells and the corresponding intestinal scrapings, of which some appear to be characteristic to human intestinal epithelium in vivo (Lenaerts et al. 2007). Nevertheless, several proteins, including transporters, were over- or underexpressed. Bourgine et al. (2012) compared the expression of 377 genes in HT29 and other intestinal cell lines that are used as in vitro models of the epithelium with the corresponding tissue biopsy. The results showed that differentiated HT29 cells and human colonic tissues do not appear to be significantly different.
The use of HT29 as in vitro model of intestinal cells has some advantages and limitations that have been summarized by Zweibaum et al. (2011). This cell line in its differentiated phenotype is similar to small intestine enterocytes with respect their structure, and the presence of brush border-associated hydrolases and the time course of the differentiation process which is also comparable to that found in the small intestine. In addition, the amount of villin expressed in differentiated HT29 cells is close to the value observed for normal freshly prepared colonocytes. However, these cells also have some limitations, because (1) they are malignant cells with a high rate of glucose consumption and an impairment of the metabolism of glucose; (2) although they mimic some characteristics of small intestine enterocytes, they are colonic cells; (3) they cannot be compared with enterocytes from normal colon since they express brush border-associated hydrolases; (4) but they cannot be compared with absorptive enterocytes because not all hydrolases are present (e.g. lactase and maltase-glucoamylase are absent) and the ion transport properties are different. It has been postulated that these cells are close to human fetal colonic cells because of the type of hydrolases present and the intracellular concentration of glycogen accumulated (Hekmati et al. 1990).
Regarding the expression of cell surface receptors, it has been reported that differentiated HT29 cells express several receptors for peptides, such as vasoactive intestinal peptide, or insulin but also for non-peptide substances. In general, the receptors found in this cell line have their equivalent in normal intestinal cells, except for the receptor to neurotensin, which has been characterised in HT29 cells (Kitabgi et al. 1980), but it is not detectable in normal human colonic epithelium. On the contrary, receptors for peptide YY or neuropeptide Y which are well characterised in normal small intestine epithelial cells have not been reported in HT29. It has to be taken into account that these receptors are located at the small intestine and that HT29 are of colonic origin. It is also remarkable that when using cell cultures, the expression of a particular receptor may depend on the degree of cell differentiation and the growing conditions. Recently, the presence of opioid, serotonin, muscarinic, PPARβ/δ receptors has been reported in this cell line (Zoghbi et al. 2006; Ataee et al. 2010; Belo et al. 2011; Foreman et al. 2011). Information on the studies on receptors carried out in this cell line can be found at Zweibaum et al. (2011).
5 General Protocol for HT29-MTX Cells
5.1 Cell Maintenance Protocol
HT29-MTX cells are cultured using DMEM medium with high glucose (25 mM), without sodium pyruvate, but with GlutaMAX® (Gibco, Paisley, UK), 10 % of heat-inactivated fetal bovine serum and penicillin/streptomycin (100 units/mL of penicillin and 100 μg/mL of streptomycin) in a humidified incubator with 5 % CO2 atmosphere at 37 °C. Culture medium is changed every 2 days from the second day after seeding, and cells are harvested in the logarithmic phase of growth after reaching 80–90 % confluency by 0.05 % trypsin/EDTA. Cells are seeded at 2.4 × 104 cells per cm2 in 75 cm2 culture flasks. In these conditions, cells would be confluent at day 6–7 of culture.
5.2 Experimental Protocol for Test Compounds
5.2.1 Study of the Mucin-Stimulating Activity
Cells are seeded in 12-well plates at a density of 5 × 105 cells per well and experiments are performed 21 days after confluency to ensure that the proportion of cells differentiated that express mucus reaches their maximum (Lesuffleur et al. 1993). Culture media is changed every 2 days until 24 h prior to the experiment, when culture media is replaced by serum- and antibiotic-free media (DMEM GlutaMAX®) to minimize any extraneous interference. On the day of the experiment, cells are rinsed twice with PBS and treated with tested compounds dissolved in serum- and antibiotic-free medium for different incubation times at 37 °C in a 5 % CO2 humidified atmosphere. After treatment, cell supernatants are collected for the determination of mucins, and adhered cells are lysed to obtain the total RNA according to the RNA isolation kit instructions. Mucin secretion is determined in cell supernatants by applying an enzyme-linked lectin assay, based on the strong binding between wheat germ agglutinin and goblet cell mucins (Campo et al. 1988). Standard curves prepared with porcine mucin are employed, and mucin gene expression is evaluated after isolating and reverse-transcribing the RNA from cell lysates. A quantitative PCR assay is performed with specific primers for MUC5AC, the HT29-MTX major secreted mucin gene (Martínez-Maqueda et al. 2013a). Cells are employed between passages 12 and 24 without remarkable basal differences.
5.2.2 Evaluation of Transepithelial Absorption by Transwell® Inserts
Transwell® inserts provides a reliable model once the integrity of the cell monolayer is verified. HT29-MTX cells are seeded in 12-well Transwell® inserts at a density of 5 × 105 cells per well and medium is changed every 2 days. Cells are cultured for at least 21 days to ensure a suitable cell differentiation. Prior to the experiment, the integrity of the cell monolayers is verified by measuring the transepithelial electrical resistance (TEER) with a cell electrical resistance system. Only monolayers with initial TEER values above 200 Ω cm−2 must be used. On the day of the experiment, cells are rinsed with Hanks’ balanced salt solution (HBSS) and test compounds are dissolved in culture medium, which are added to the apical side of the cell layer (HBSS to the basolateral chamber). According to Foltz et al. (2008), cells are maintained at 37 °C in a 5 % CO2 humidified atmosphere. Transport assay is initiated by adding 0.5 mL of transport medium, containing compounds of interest to the apical compartment. Samples (100 μL) are taken from the basolateral compartment and replaced by transport medium. After the end of the experiment, TEER measurement is repeated and permeability values are only calculated from experiments in which the second TEER value is at least 75 % of the initial TEER value. Fluorescein flux can be measured as a second parameter of monolayer integrity. Monolayers are deemed intact when TEER values were above 200 Ω cm−2, and the permeability coefficients of the paracellular transport marker fluorescein is less than 1 × 10−6 cm s−1. Apical and basolateral solutions can be collected and analyzed by RP-HPLC–MS/MS to evaluate the permeability, including permeability coefficients as indicated by Quirós et al. (2008) and Contreras et al. (2012), and degradation of tested compounds.
6 Experimental Read Out
6.1 Functionality Studies
HT29 cells have been widely employed to assess the potential anticancer effect of different food compounds, and their gastrointestinal digests, on the basis of the carcinoma origin of this cell line. An illustrative example is found in the study of the antiproliferative activity of the in vitro gastrointestinal digest of sea cucumber wall (Pérez-Vega et al. 2013). Another representative study is found in the assessment of the cytotoxic activity of different extracts from “Racimo” tomato variety and simulated digests against HT29 cell growth, including also the evaluation of the selectivity of the toxic effect against cancer cells (Guil-Guerrero et al. 2011). Other activities have been widely studied on HT29 cells or derived lines, such as immunomodulatory, antioxidant or barrier protective properties. For instance, milk and soy ferments with different strains of lactic acid bacteria (LAB) were evaluated on human intestinal epithelial cells, including HT29 cells, to test their immunomodulatory activity (Wagar et al. 2009). The study was carried out on cells treated with TNF-α and the production of IL-8 was evaluated in the cell supernatant by a commercial enzyme-linked immunosorbent assay. Another investigation focused on the study of the immunomodulatory activity of cereal β-glucan preparations on both HT29 and Caco-2 cells (Rieder et al. 2011). In vitro data of antioxidative activity for food compounds is often presented together with genotoxic tests performed with HT29 cells. Among others, the comet assay has become one of the standard methods for assessing DNA integrity (Collins 2004). Ferguson et al. (2005) demonstrated both in vitro antioxidant activity and antigenotoxic effect in HT29 cells by using a comet assay for two hydroxycinnamic acids with an important percentage in certain plant foods, as spinach or cereals. Interestingly, the protection of resveratrol and quercetin against exogenous pro-oxidative damage was determined in HT29 cells with induced oxidative stress by addition of fatty acid hydroperoxides (Kaindl et al. 2008). HT29-derived cell lines constitute a valuable tool related to the strengthening of the intestinal mucus barrier to study the mucin-stimulating activity of food compounds. The protocol described above has been recently applied to asses the mucin-stimulating effect of certain milk protein hydrolysates and derived peptides (Martínez-Maqueda et al. 2012, 2013a, b). HT29-MTX cells and an analogous experimental design was also implemented to evaluate the regulation of the mucin production by the β-casomorphin-7, a μ-opioid peptide derived from bovine milk (Zoghbi et al. 2006), or by a yoghurt peptide pool (Plaisancié et al. 2013). Other HT29 derived cell lines have been employed due to their capability to form a mucus-layer, e.g. HT29/B6 cells differentiated in a glucose-free culture (Kreusel et al. 1991). Hering et al. (2011) evaluated the expression of tight junction proteins and the related intestinal barrier-protection in HT29/B6 cells, under the effect of TGF-β, a whey protein component. Likewise, monoterpene d-limonene, naturally occurring in the rind of citrus fruit, showed a significant increase on the transepithelial resistance in HT29/B6 cells (D’Alessio et al. 2013).
6.2 Transport Studies
The HT29 cell line is not a particularly suitable model for transport studies, but it has been used for comparison with other lines or as co-culture. Monolayers of HT29-MTX cells have been previously used as a permeability model, for example for studying the effect of mucus on the permeation of drugs. However, in most cases, transport studies and permeation enhancement studies are performed with co-cultures of Caco-2 and HT29-MTX as an accurate model to take into account the mucus layer (Yuan et al. 2013).
It has been reported that casein phosphopeptides (CPPs) form aggregated complexes with calcium phosphate and induce Ca2+ influx into HT29 cells that have been shown to be differentiated in culture. Taking into account that upon differentiation HT29 cells elicit a transient rise in Ca2+, this model is being used to explore the molecular mechanism by which CPPs elicit their biological activity (Gravaghi et al. 2007). Similarly, the HT29 cell line is a useful model to study key players involved in intestinal iron absorption because it endogenously expresses a number of genes known to be involved in iron transport in the intestine. Using this model, it has been suggested that the hemochromatosis protein HFE can have multiple roles in maintaining iron homeostasis depending on the availability of other proteins (Davies and Enns 2004).
HT29 cells have also been applied to the study of the receptor and internalization transporter of toxins. As for the Clostridium botulinum C16S toxin, it was reported that the receptor on the HT29 cell surface targeted is an O-linked sugar chain of mucin-like glycoproteins (Nishikawa et al. 2004).
6.3 Microorganisms Survival, Adhesion or Invasion
The HT29 cells represent a well characterised model to study the intestinal epithelial response to bacterial infection. This cell line expresses the features of enterocytes and is useful for attachment and mechanistic studies. Mucin secretion by these cells is of importance because the mucus layer has been suggested to play a role in modulating the adhesion of live organisms to the epithelial surface as well as bacterial components such as LPS (Otte and Podolsky 2004).
The mechanisms underlying enteropathogenesis are one of the most important applications of this cell model. Studies on attachment, adherence and/or internalization of several pathogens such as Entamoeba histolytica, Salmonella enteriditis, Escherichia coli, Clostridium difficile, Campylobacter jejuni, Clostridium butyricum or Shigella disenteriae, in some cases with food components intended to limit their access to cells have been reported. For example, in the study of Nickerson and McDonald (2012) it was shown that exposure to maltodextrin induced type I pilus expression, which was required for enhanced biofilm formation by E. coli in Crohn’s disease patients. Comparison of the results with those found in the HT29 cell model was used to suggest a novel mechanism of epithelial cell adhesion that can contribute to disease susceptibility.
Studies intended to evaluate the beneficial role of probiotics in the human host are another application of the cell model. For the study of probiotic adhesion, the HT29 cell line and some HT29-derived lines have been used to study the molecular mechanisms underpinning host-microbe interactions or the enteric adaptation features of Bifidobacterium strains, sometimes in the presence of food components as milk oligosaccharides (Kavanaugh et al. 2013). Probiotic delivery systems by coating biopolymers are also evaluated by using colonic epithelial models that are intended to mimic the colonic mucosa. The intestinal mucus offers numerous ecological advantages for both resident microbiotic bacteria and some pathogenic bacteria present within the lumen and in the intestinal epithelium. It can provide nutrients for bacterial growth, thus promoting intestinal colonization by the adhering bacteria which have the ability to survive and multiply in the outer regions of the mucus layer (Liévin-Le Moal and Servin 2006).
In response to enteropathogens infection, the intestinal epithelium releases the chemokine IL-8 and other pro-inflammatory molecules that provoke an acute inflammatory response. For the protection against enteropathogens infections, the possibility of using food supplements containing probiotic bacteria is increasingly considered. Selected strains have shown to be able to survive under gastrointestinal challenges, while they were shown to adhere to human epithelial intestinal cell monolayers (Caco-2 and HT29), thereby preventing the establishment of enteric pathogens as E. coli and Cronobacter sakazakii (Serafini et al. 2013). Furthermore, many probiotic strains have been assessed for their immunomodulatory activity on IL-8 production by HT29 cells, protecting them from an acute inflammatory response (Candela et al. 2008).
7 Conclusions
Despite several limitations of the human colon carcinoma cell lines, they are valuable tools to study several aspects related with food digestion and bioavailability of food compounds. HT29 cell line and some derived cell lines thereof are of interest to study food component absorption, especially when used in co-culture with other epithelial cell lines. Although the quantitative results can be questioned in comparison with those obtained in vivo, these in vitro models allow to identify modifications that the food compound may suffer during absorption and these results will help to perform further in vivo studies in animal or humans. The use of these human cells also offers a valuable opportunity to perform studies when a variance in response between different species is observed. For instance, the mucus secreting phenotype of HT29 cell line has proved to be a valuable model to screen food compounds or bacteria which may influence mucus secreting properties in the gut. Finally, it has provided results about the mechanisms by which microbes adhere, invade and signal to the host, as well as, to examine the mammalian cell response. Altogether, they are a complementary tool to the in vivo and ex vivo strategies to study food digestion and the effect of food components on the gut.
References
Ataee R, Ajdary S, Zarrindast M, Rezayat M, Hayatbakhsh MR (2010) Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. J Cancer Res Clin Oncol 136(10):1461–1469. doi:10.1007/s00432-010-0801-3
Augeron C, Laboisse CL (1984) Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res 44(9):3961–3969
Belo A, Cheng KR, Chahdi A, Shant J, Xie GF, Khurana S, Raufman JP (2011) Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol 300(5):G749–G760. doi:10.1152/ajpgi.00306.2010
Bourgine J, Billaut-Laden I, Happillon ML, Lo-Guidice J-M, Maunoury V, Imbenotte M, Broly F (2012) Gene expression profiling of systems involved in the metabolism and the disposition of xenobiotics: comparison between human intestinal biopsy samples and colon cell lines. Drug Metab Dispos 40(4):694–705. doi:10.1124/dmd.111.042465
Campo E, Condom E, Palacín A, Quesada E, Cardesa A (1988) Lectin binding patterns in normal and neoplastic colonic mucosa—a study of dolichos biflorus agglutinin, peanut agglutinin, and wheat germ agglutinin. Dis Colon Rectum 31(11):892–899. doi:10.1007/BF02554856
Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 125(3):286–292. doi:10.1016/j.ijfoodmicro.2008.04.012
Chen TR, Drabkowski D, Hay RJ, Macy M, Peterson W Jr (1987) WiDr is a derivative of another colon adenocarcinoma cell line, HT-29. Cancer Genet Cytogenet 27(1):125–134
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26(3):249–261. doi:10.1385/MB:26:3:249
Contreras MM, Sancho AI, Recio I, Mills C (2012) Absorption of casein antihypertensive peptides through an in vitro model of intestinal epithelium. Food Digestion 3(1–3):16–24. doi:10.1007/s13228-012-0020-2
D’Alessio PA, Ostan R, Bisson J, Schulzke JD, Ursini MV, Béné MC (2013) Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci 92(24–26):1151–1156. doi:10.1016/j.lfs.2013.04.013
Davies PS, Enns CA (2004) Expression of the hereditary hemochromatosis protein HFE increases ferritin levels by inhibiting iron export in HT29 cells. J Biol Chem 279(24):25085–25092. doi:10.1074/jbc.M400537200
Desai S, Kumar A, Laskar S, Pandey BN (2013) Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 61(1):54–62. doi:10.1016/j.cyto.2012.08.022
Ferguson LR, Zhu S, Harris PJ (2005) Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol Nutr Food Res 49(6):585–593. doi:10.1002/mnfr.200500014
Fitzgerald RC, Omary MB, Triadafilopoulos G (1997) Acid modulation of HT29 cell growth and differentiation. An in vitro model for Barrett’s oesophagus. J Cell Sci 110(5):663–671
Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J (ed) Human tumor cell in vitro, 1st edn. Springer, New York, pp 115–159. doi:10.1007/978-1-4757-1647-4_5
Foltz M, Cerstiaens A, van Meensel A, Mols R, van der Pijl PC, Duchateau GSMJE, Augustijns P (2008) The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 29(8):1312–1320. doi:10.1016/j.peptides.2008.03.021
Foreman JE, Chang W-CL, Palkar PS, Zhu B, Borland MG, Williams JL, Kramer LR, Clapper ML, Gonzalez FJ, Peters JM (2011) Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol Carcinog 50(11):884–900. doi:10.1002/mc.20757
Gravaghi C, Del Favero E, Cantu L, Donetti E, Bedoni M, Fiorilli A, Tettamanti G, Ferraretto A (2007) Casein phosphopeptide promotion of calcium uptake in HT-29 cells—relationship between biological activity and supramolecular structure. FEBS J 274(19):4999–5011. doi:10.1111/j.1742-4658.2007.06015.x
Guil-Guerrero JL, Ramos-Bueno R, Rodríguez-García I, López-Sánchez C (2011) Cytotoxicity screening of several tomato extracts. J Med Food 14(1–2):40–45. doi:10.1089/jmf.2010.0051
Hekmati M, Polak-Charcon S, Ben Shaul Y (1990) A morphological study of a human adenocarcinoma cell line (HT29) differentiating in culture. Similarities to intestinal embryonic development. Cell Differ Dev 31(3):207–218. doi:10.1016/0922-3371(90)90133-H
Hering NA, Andres S, Fromm A, van Tol EA, Amasheh M, Mankertz J, Fromm M, Schulzke JD (2011) Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr 141(5):783–789. doi:10.3945/jn.110.137588
Huet C, Sahuquillo-Merino C, Coudrier E, Louvard D (1987) Absorptive and mucus‐secreting subclones isolated from a multipotent intestinal cell line (HT‐29) provide new models for cell polarity and terminal differentiation. J Cell Biol 105(1):345–358
Kaindl U, Eyberg I, Rohr-Udilova N, Heinzle C, Marian B (2008) The dietary antioxidants resveratrol and quercetin protect cells from exogenous pro-oxidative damage. Food Chem Toxicol 46(4):1320–1326. doi:10.1016/j.fct.2007.09.002
Kavanaugh DW, O’Callaghan J, Buttó LF, Slattery H, Lane J, Clyne M, Kane M, Joshi L, Hickey RM (2013) Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS One 8(6):e67224. doi:10.1371/journal.pone.0067224
Kitabgi P, Poustis C, Granier C, Van Rietschoten J, Rivier J, Morgat JL, Freychet P (1980) Neurotensin binding to extraneural and neural receptors: comparison with biological activity and structure-activity relationships. Mol Pharmacol 18(1):11–19
Kreusel K, Fromm M, Schulzke J, Hegel U (1991) Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol Cell Physiol 261(4):C574–C582
Langerholc T, Maragkoudakis PA, Wollgast J, Gradisnik L, Cencic A (2011) Novel and established intestinal cell line models—an indispensable tool in food science and nutrition. Trends Food Sci Technol 22(Suppl 1):S11–S20. doi:10.1016/j.tifs.2011.03.010
Lenaerts K, Bouwman F, Lamers W, Renes J, Mariman E (2007) Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics 8:91. doi:10.1186/1471-2164-8-91
Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A (1990) Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res 50(19):6334–6343
Lesuffleur T, Porchet N, Aubert J, Swallow D, Gum JR, Kim YS, Zweibaum A (1993) Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci 106(3):771–783
Liévin-Le Moal V, Servin AL (2006) The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev 19(2):315–337. doi:10.1128/CMR. 19.2.315-337.2006
Lipps C, May T, Hauser H, Wirth D (2013) Eternity and functionality-rational access to physiologically relevant cell lines. Biol Chem 394(12):1637–1648. doi:10.1515/hsz-2013-0158
Maoret JJ, Font J, Augeron C, Codogno P, Bauvy C, Aubery M, Laboisse CL (1989) A mucus-secreting human colonic cancer cell line. Purification and partial secretion of the secreted mucins. Biochem J 258(3):793–799
Martínez-Maqueda D, Miralles B, De Pascual-Teresa S, Reverón I, Muñoz R, Recio I (2012) Food-derived peptides stimulate mucin secretion and gene expression in intestinal cells. J Agric Food Chem 60(35):8600–8605. doi:10.1021/jf301279k
Martínez-Maqueda D, Miralles B, Ramos M, Recio I (2013a) Effect of β-lactoglobulin hydrolysate and β-lactorphin on intestinal mucin secretion and gene expression in human goblet cells. Food Res Int 54(1):1287–1291. doi:10.1016/j.foodres.2012.12.029
Martínez-Maqueda D, Miralles B, Cruz-Huerta E, Recio I (2013b) Casein hydrolysate and derived peptides stimulate mucin secretion and gene expression in human intestinal cells. Int Dairy J 32(1):13–19. doi:10.1016/j.idairyj.2013.03.010
Nickerson KP, McDonald C (2012) Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One 7(12):e52132. doi:10.1371/journal.pone.0052132
Nishikawa A, Uotsu N, Arimitsu H, Lee J-C, Miura Y, Fujinaga Y, Nakada H, Watanabe T, Ohyama T, Sakano Y, Oguma K (2004) The receptor and transporter for internalization of Clostridium botulinum type C progenitor toxin into HT-29 cells. Biochem Biophys Res Commun 319(2):327–333. doi:10.1016/j.bbrc.2004.04.183
Otte J-M, Podolsky DK (2004) Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286(4):G613–G626
Pérez-Vega JA, Olivera-Castillo L, Gómez-Ruiz JT, Hernández-Ledesma B (2013) Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J Funct Foods 5(2):869–877. doi:10.1016/j.jff.2013.01.036
Pinto M, Appay MD, Simon-Assman P, Chevalier G, Dracopoli N, Fogh J, Zweibaum A (1982) Enterocytic differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol Cell 44(2):193–196
Plaisancié P, Claustre J, Estienne M, Henry G, Boutrou R, Paquet A, Léonil J (2013) A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and paneth cells along the small intestine. J Nutr Biochem 24(1):213–221. doi:10.1016/j.jnutbio.2012.05.004
Quirós A, Dávalos A, Lasunción MA, Ramos M, Recio I (2008) Bioavailability of the antihypertensive peptide LHLPLP: transepithelial flux of HLPLP. Int Dairy J 18(3):279–286. doi:10.1016/j.idairyj.2007.09.006
Rieder A, Grimmer S, Kolset SO, Michaelsen TE, Knutsen SH (2011) Cereal β-glucan preparations of different weight average molecular weights induce variable cytokine secretion in human intestinal epithelial cell lines. Food Chem 128(4):1037–1043. doi:10.1016/j.foodchem.2011.04.010
Serafini F, Strati F, Ruas-Madiedo P, Turroni F, Foroni E, Duranti S, Milano F, Perotti A, Viappiani A, Guglielmetti S, Buschini A, Margolles A, van Sinderen D, Ventura M (2013) Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe 21:9–17. doi:10.1016/j.anaerobe.2013.03.003
Wagar LE, Champagne CP, Buckley ND, Raymond Y, Green-Johnson JM (2009) Immunomodulatory properties of fermented soy and dairy milks prepared with lactic acid bacteria. J Food Sci 74(8):M423–M430. doi:10.1111/j.1750-3841.2009.01308.x
Yuan H, Chen CY, Chai GH, Du YZ, Hu FQ (2013) Improved transport and absorption through gastrointestinal tract by pegylated solid lipid nanoparticles. Mol Pharm 10(5):1865–1873. doi:10.1021/mp300649z
Zoghbi S, Trompette A, Claustre J, El Homsi M, Garzon J, Scoazec JY, Plaisancie P (2006) Beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am J Physiol Gastrointest Liver Physiol 290(6):G1105–G1113. doi:10.1152/ajpgi.00455.2005
Zweibaum A (1986) Enterocytic differentiation of cultured human colon cancer cell lines: negative modulation by d‐glucose. In: Alvarado F, van Os CH (eds) Ion gradient‐coupled transport. Elsevier, Amsterdam, pp 345–353
Zweibaum A, Laburthe M, Grasset E, Louvard D (2011) Use of cultured cell lines in studies of intestinal cell differentiation and function. Compr Physiol (Suppl 19):223–255. doi:10.1002/cphy.cp060407
Acknowledgments
This work was supported by projects AGL2011-24643 from Ministerio de Economía y Competitividad and FP7-SME-2012-315349 (FOFIND). The authors are participants in the FA1005 COST Action INFOGEST on food digestion.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Martínez-Maqueda, D., Miralles, B., Recio, I. (2015). HT29 Cell Line. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)