Abstract
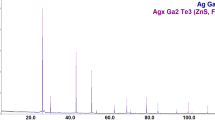
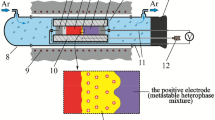
The phase equilibria of the Ag–Ga–S–AgBr system in the part GaS–Ga2S5–AgBr–Ag2S below 600 K were investigated by the modified electromotive force (EMF) method using the Ag+ catalysts as small nucleation centers of equilibrium phases. Division of the GaS–Ga2S5–AgBr–Ag2S was carried out with the participation of the following compounds Ag2S, GaS, Ga2S3, AgBr, Ag9GaS6, AgGaS2, Ag3SBr, Ag3Ga2S4Br, and Ag27Ga2S12Br9. Reactions were performed by applying electrochemical cells (ECs) with the structure: (−) IE | NE | SSE | R{Ag+} | PE | IE (+), where IE is the inert electrode (graphite powder), NE is the negative electrode (silver powder), SSE is the solid-state electrolyte (glassy Ag3GeS3Br), PE is the positive electrode, R{Ag+} is the region of Ag+ diffusion into PE. The measured EMF and temperature values of ECs were used to determine the standard thermodynamic functions of the compounds Ag3Ga2S4Br and Ag27Ga2S12Br.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Moroz M, Tesfaye F, Demchenko P, Prokhorenko M, Prokhorenko S, Reshetnyak O (2021) Non-activation synthesis and thermodynamic properties of ternary compounds of the Ag–Te–Br system. Thermochim Acta 698:178862(1)–(7). https://doi.org/10.1016/j.tca.2021.178862
Moroz M, Tesfaye F, Demchenko P, Kordan V, Prokhorenko M, Mysina O, Reshetnyak O, Gladyshevskii R (2023) Synthesis, thermodynamic properties, and structural characteristics of multicomponent compounds in the Ag–Ni–Sn–S system. JOM 75:2016–2025. https://doi.org/10.1007/s11837-023-05784-9
Ivashchenko I, Kozak V, Gulay L, Olekseyuk I (2022) Crystal structure of AgGa2Se3Cl(Br) compounds. Proc Shevchenko Sci Soc Ser Chem Sci LXX:62–68. https://doi.org/10.37827/ntsh.chem.2022.70.062
Range K-J, Handrick K (1988) Neue 1320637-Verbindungen/New 1320637 compounds. Z Naturforsch B 43:240–242. https://doi.org/10.1515/znb-1988-0218
Brandt G, Krämer V (1976) Phase investigations in the silver-gallium-sulphur system. Mater Res Bull 11:1381–1388. https://doi.org/10.1016/0025-5408(76)90049-0
Chbani N, Loireau-Lozac’h A-M, Rivet J, Dugué J (1995) Système pseudo-ternaire Ag2S-Ga2S3-GeS2: diagramme de phases—domaine vitreux. J Solid State Chem 117:189–200. https://doi.org/10.1006/jssc.1995.1262
Ibragimova GI, Shikhiyev YuM, Babanly MB (2006) Solid phase equlibria in Ag-Ga-S (Se, Te) systems and thermodynamic properties of ternary phases. Chem Probl 1:23–28
Lin S, Li W, Bu Z, Gao B, Li J, Pei Y (2018) Thermoelectric properties of Ag9GaS6 with ultralow lattice thermal conductivity. Mater Today Phys 6:60–67. https://doi.org/10.1016/j.mtphys.2018.09.001
Hellstrom E, Schoonman J (1980) Silver ionic and electronic conductivity in Ag9GaS6. Solid State Ionics 1:199–210. https://doi.org/10.1016/0167-2738(80)90004-1
Lin S, Li W, Pei Y (2021) Thermally insulative thermoelectric argyrodites. Mater Today 48:198–213. https://doi.org/10.1016/j.mattod.2021.01.007
Asadov MM, Mustafaeva SN (2015) X-ray dosimetry of an AgGaS2 single crystal. Bull Russ Acad Sci Phys 79:1113–1117. https://doi.org/10.3103/S106287381509004X
Laksari S, Chahed A, Abbouni N, Benhelal O, Abbar B (2006) First-principles calculations of the structural, electronic and optical properties of CuGaS2 and AgGaS2. Comput Mater Sci 38:223–230. https://doi.org/10.1016/j.commatsci.2005.12.043
Mouacher R, Seddik T, Rezini B, Haq B, Batouche M, Ugur G, Ugur S, Belfedal A (2022) First-principles calculations of electronic and optical properties of AgGa1-xTlxS2 alloys: analyses and design for solar cell applications. J Solid State Chem 309:122996. https://doi.org/10.1016/j.jssc.2022.122996
Palazon F (2022) Metal chalcohalides: next generation photovoltaic materials? Sol RRL 6:2100829. https://doi.org/10.1002/solr.202100829
Piasecki M, Myronchuk GL, Parasyuk OV, Khyzhun OY, Fedorchuk AO, Pavlyuk VV (2017) Synthesis, structural, electronic and linear electro-optical features of new quaternary Ag2Ga2SiS6 compound. J Solid State Chem 246:363–371. https://doi.org/10.1016/j.jssc.2016.12.011
Kim J-H, Kim B-Y, Jang E-P, Yoon S-Y, Kim K-H (2018) Synthesis of widely emission-tunable Ag–Ga–S and its quaternary derivative quantum dots. Chem Eng J 347:791–797. https://doi.org/10.1016/j.cej.2018.04.167
Wei J, Hu Z, Zhou W, Qiu Y, Dai H (2021) Emission tuning of highly efficient quaternary Ag-Cu-Ga-Se/ZnSe quantum dots for white light-emitting diodes. J Colloid Interface Sci 602:307–315. https://doi.org/10.1016/j.jcis.2021.05.110
Azhniuk Y, Lopushanska B, Selyshchev O, Havryliuk Y, Pogodin A (2022) Synthesis and optical properties of Ag–Ga–S quantum dots. Phys Status Solidi B 259:2100349. https://doi.org/10.1002/pssb.202100349
Valakh M, Litvinchuk AP, Havryliuk Y, Yukhymchuk V, Dzhagan V (2023) Raman- and infrared-active phonons in nonlinear semiconductor AgGaGeS4. Crystals 13:148. https://doi.org/10.3390/cryst13010148
Thirumoorthy M, Ramesh K (2021) Characteristics of pulse electrodeposited AgGaS2 thin films for photovoltaic application. Mater Today Proc 47:1847–1854. https://doi.org/10.1016/j.matpr.2021.03.410
Moroz MV, Demchenko PYu, Prokhorenko MV, Reshetnyak OV (2017) Thermodynamic properties of saturated solid solutions of the phases Ag2PbGeS4, Ag0.5Pb1.75GeS4 and Ag6.72Pb0.16Ge0.84S5.20 of the Ag-Pb-Ge-S system determined by EMF method. J Phase Equilibria Diffus 38:426–433. https://doi.org/10.1007/s11669-017-0563-6
Moroz MV, Prokhorenko MV, Reshetnyak OV, Demchenko PYu (2017) Electrochemical determination of thermodynamic properties of saturated solid solutions of Hg2GeSe3, Hg2GeSe4, Ag2Hg3GeSe6, and Ag1.4Hg1.3GeSe6 compounds in the Ag–Hg–Ge–Se system. J Solid State Electrochem 21:833–837. https://doi.org/10.1007/s10008-016-3424-z
Moroz MV, Demchenko PYu, Mykolaychuk OG, Akselrud LG, Gladyshevskii RE (2013) Synthesis and electrical conductivity of crystalline and glassy alloys in the Ag3GeS3Br-GeS2 system. Inorg Mater 49:867–871. https://doi.org/10.1134/S0020168513090100
Moroz MV, Prokhorenko MV (2014) Thermodynamic properties of the intermediate phases of the Ag-Sb-Se system. Russ J Phys Chem A 88:742–746. https://doi.org/10.1134/S0036024414050203
Moroz M, Tesfaye F, Demchenko P, Prokhorenko M, Lindberg D, Reshetnyak O, Hupa L (2018) Phase equilibria and thermodynamics of selected compounds in the Ag–Fe–Sn–S system. J Electron Mater 47:5433–5442. https://doi.org/10.1007/s11664-018-6430-3
Moroz MV, Prokhorenko MV, Rudyk BP (2014) Thermodynamic properties of phases of the Ag-Ge-Te system. Russ J Electrochem 50:1177–1181. https://doi.org/10.1134/S1023193514120039
Prokhorenko MV, Moroz MV, Demchenko PYu (2015) Measuring the thermodynamic properties of saturated solid solutions in the Ag2Te-Bi-Bi2Te3 system by the electromotive force method. Russ J Phys Chem A 89:1330–1334. https://doi.org/10.1134/S0036024415080269
Moroz M, Tesfaye F, Demchenko P, Prokhorenko M, Kogut Y, Pereviznyk O, Prokhorenko S, Reshetnyak O (2020) Solid-state electrochemical synthesis and thermodynamic properties of selected compounds in the Ag–Fe–Pb–Se system. Solid State Sci 107:106344(1)–(9). https://doi.org/10.1016/j.solidstatesciences.2020.106344
Babanly M, Yusibov Y, Babanly N (2011) The EMF method with solid-state electrolyte in the thermodynamic investigation of ternary copper and silver chalcogenides. In: Kara S (ed). InTech, pp 57–78. https://doi.org/10.5772/28934
Mammadov FM, Amiraslanov IR, Imamaliyeva SZ, Babanly MB (2019) Phase relations in the FeSe–FeGa2Se4–FeIn2Se4 system: refinement of the crystal structures of FeIn2Se4 and FeGaInSe4. J Phase Equilibria Diffus 40:787–796. https://doi.org/10.1007/s11669-019-00768-2
Hasanova GS, Aghazade AI, Babanly DM, Imamaliyeva SZ, Yusibov YA, Babanly MB (2021) Experimental study of the phase relations and thermodynamic properties of Bi-Se system. J Therm Anal Calorim 147:6403–6414. https://doi.org/10.1007/s10973-021-10975-0
Gravetter FJ, Wallnau LB (2017) Statistics for the behavioral sciences, 10th edn. Cengage Learning, Australia and United States
Babanly NB, Orujlu EN, Imamaliyeva SZ, Yusibov YA, Babanly MB (2019) Thermodynamic investigation of silver-thallium tellurides by EMF method with solid electrolyte Ag4RbI5. J Chem Thermodyn 128:78–86. https://doi.org/10.1016/j.jct.2018.08.012
Imamaliyeva SZ, Musayeva SS, Babanly DM, Jafarov YI, Taghiyev DB, Babanly MB (2019) Determination of the thermodynamic functions of bismuth chalcoiodides by EMF method with morpholinium formate as electrolyte. Thermochim Acta 679:178319. https://doi.org/10.1016/j.tca.2019.178319
Barin I (1995) Thermochemical data of pure substances. VCH, Weinheim
Moroz MV, Prokhorenko MV, Prokhorenko SV (2015) Determination of thermodynamic properties of Ag3SBr superionic phase using EMF technique. Russ J Electrochem 51:886–889. https://doi.org/10.1134/S1023193515090098
Acknowledgements
The present work was financed partially by the grant of the Ministry of Education and Science of Ukraine No 0123U101857 “Physico-chemistry of functional nanomaterials for electrochemical systems”, international projects: #HX-010123 from “Materials Phases Data System, Viznau, Switzerland” and the Simons Foundation (Award Number: 1037973). This work was partly funded by the K.H. Renlund Foundation under the project “Innovative e-waste recycling processes for greener and more efficient recoveries of critical metals and energy” at Åbo Akademi University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Moroz, M. et al. (2024). Phase Equilibria and Thermodynamic Properties of Selected Compounds in the Ag–Ga–S–AgBr System for Modern Application in Energy Conversion Devices. In: Iloeje, C., et al. Energy Technology 2024. TMS 2024. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50244-6_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-50244-6_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50243-9
Online ISBN: 978-3-031-50244-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)