Abstract
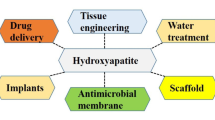
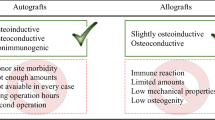
It is well-known that bone is one of the most commonly replaced organs worldwide after a blood transfusion and therefore, the development of different strategies is necessary for treating bone defects. Based on this concept, bone surgical problems have been solved. Thanks to biomaterials, which involve calcium phosphates/silicates, porous metals, bioactive glasses, glass–ceramics, and synthetic polymers. Notably, hydroxyapatite (HA) is one of the calcium phosphate family, which possesses marvelous ability to integrate with hard tissues without triggering foreign body reactions and consequently, save patients from the possible undesirable complications. It is meaningful to mention that in spite of the attractive applications of HA in medicine, it has other important applications in many fields like agriculture, chemistry, and the environment. This chapter briefly discusses the different bone grafting materials, and their defects, 3D bioprinting of scaffolds, the definition and types of biomaterials, host responses to biomaterials, different methods for producing HA, and the different properties and applications of HA. Noteworthy, one can say that the preparation of HA with certain characteristics is very complicated due to the potential production of toxic intermediary phases that may occur during the synthesis process. Meanwhile, further investigations and descriptions of the HA structure and fictionalization could be achieved with molecular modeling at different levels.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
G. Turnbull, J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li, W. Shu, 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 3, 278–314 (2018). https://doi.org/10.1016/j.bioactmat.2017.10.001
B. Dhandayuthapani, Y. Yoshida, T. Maekawa, D.S. Kumar, Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci. 2011, 1–19 (2011). https://doi.org/10.1155/2011/290602
E. García-Gareta, M.J. Coathup, G.W. Blunn, Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 81, 112–121 (2015). https://doi.org/10.1016/j.bone.2015.07.007
S.V. Murphy, A. Atala, 3D bioprinting of tissues and organs. Nat. Biotechnol. 32(8), 773–785 (2014). https://doi.org/10.1038/nbt.2958
A. Shafiee, A. Atala, Printing technologies for medical applications. Trends Mol. Med. 22(3), 254–265 (2016). https://doi.org/10.1016/j.molmed.2016.01.003
J. Lu, C. Yan, 3D printing of scaffolds for tissue engineering. IntechOpen (2018)
J. Cui et al., Polydopamine-functionalized polymer particles as templates for mineralization of hydroxyapatite: biomimetic and in vitro bioactivity. RSC Adv. 6(8), 6747–6755 (2016). https://doi.org/10.1039/C5RA24821C
R.E. Saunders, B. Derby, Inkjet printing biomaterials for tissue engineering: bioprinting. Int. Mater. Rev. 59(8), 430–448 (2014). https://doi.org/10.1179/1743280414Y.0000000040
S. Bose, S. Vahabzadeh, A. Bandyopadhyay, Bone tissue engineering using 3D printing. Mater. Today 16(12), 496–504 (2013). https://doi.org/10.1016/j.mattod.2013.11.017
V. Keriquel et al., In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 7(1), 1778 (2017). https://doi.org/10.1038/s41598-017-01914-x
L. Koch, M. Gruene, C. Unger, B. Chichkov, Laser assisted cell printing. Curr. Pharm. Biotechnol. 14(1), 91–97 (2013). https://doi.org/10.2174/138920113804805368
Y. Yu et al., Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 6, 28714 (2016). https://doi.org/10.1038/srep28714
J. Zhang, W. Liu, V. Schnitzler, F. Tancret, J.M. Bouler, Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 10, 1035–1049 (2014). https://doi.org/10.1016/j.actbio.2013.11.001
M. Catauro, F. Bollino, Advanced glass-ceramic materials for biomedical applications. J. Bone Rep. Recommend. 3, 1–3 (2017). https://doi.org/10.4172/2469-6684.100035
R.A. Youness, M.A. Taha, A.A. El-Kheshen, N. El-Faramawy, M. Ibrahim, In vitro bioactivity evaluation, antimicrobial behavior and mechanical properties of cerium-containing phosphate glasses. Mater. Res. Expr. 6, 1–13 (2019). https://doi.org/10.4172/2469-6684.100035
E.M.A. Khalil, R.A. Youness, M.S. Amer, M.A. Taha, Mechanical properties, in vitro and in vivo bioactivity assessment of Na2O-CaO-P2O5-B2O3-SiO2 glass-ceramics. Ceram. Int. 44, 7867–7876 (2018). https://doi.org/10.1016/j.ceramint.2018.01.222
R.A. Youness, M.A. Taha, M. Ibrahim, A. El-Kheshen, FTIR spectral characterization, mechanical properties and antimicrobial properties of La-doped phosphate-based bioactive glasses. Silicon 10, 1151–1159 (2018). https://doi.org/10.1007/s12633-017-9587-0
S.M. Abo-Naf, E.M. Khalil, E.M. El-Sayed, H.A. Zayed, R.A. Youness, In vitro bioactivity evaluation, mechanical properties and microstructural characterization of Na2O-CaO-B2O3-P2O5 glasses. Spectrochim. Acta A 144, 88–98 (2015). https://doi.org/10.1016/j.saa.2015.02.076
M.A. Taha, R.A. Youness, M.F. Zawrah, Phase composition, sinterability and bioactivity of amorphous nano-CaO-SiO2-CuO powder synthesized by sol-gel technique. Ceram. Int. 46, 24462–24471 (2020). https://doi.org/10.1016/j.ceramint.2020.06.231
W.S. AbuShanab, E.B. Moustafa, M.A. Taha, R.A. Youness, Synthesis and structural properties characterization of titnia/zirconia/calcium silicate nanocomposites for biomedical applications. Appl. Phys. A 126, 1–12 (2020). https://doi.org/10.1007/s00339-020-03975-8
G. Kaur, G. Pickrell, G. Kimsawatde, D. Homa, H.A. Allbee, N. Sriranganathan, Synthesis, cytotoxicity, and hydroxyapatite formation in 27-Tris-SBF for sol-gel based CaO-P2O5-SiO2-B2O3-ZnO bioactive glasses. Sci. Rep. 4, 1–14 (2013). https://doi.org/10.1038/srep04392
R. Ravarian, F. Moztarzadeh, M.S. Hashjin, S.M. Rabiee, P. Khoshakhlagh, M. Tahriri, Synthesis, characterization and bioactivity investigation of bioglass/hydroxyapatite composite. Ceram. Int. 36, 291–297 (2010). https://doi.org/10.1016/j.ceramint.2009.09.016
K. Labgairi, A. Borji, M. Kaddami, A. Jourani, Kinetic study of calcium phosphate precipitation in the system H3PO4–Ca(OH)2–H2O at 30°C. Int. J. Chem. Eng. 2020, 1–9 (2020). https://doi.org/10.1155/2020/2893298
E. Mariani, G. Lisignoli, R.M. Borzì, L. Pulsatelli, Biomaterials: foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 20, 1–42 (2019). https://doi.org/10.3390/ijms20030636
R.M. Berne, M.N. Levy, B.M. Koeppen, B.A. Stanton, Berne and Levy Physiology, 6th edn. (Mosby, St. Louis, 2009)
A.C. Guyton, J.E. Hall, Textbook of Medical Physiology, 12th edn. (Elsevier Saunders, Philadelphia, 2010)
A.B.H. Yoruç, B.C. Şener, Biomaterials, a roadmap of biomedical engineers and milestones, ed. by Sadik Kara (InTech, 2012). ISBN: 978-953-51-0609-8
F.O. Costa, S. Takenaka-Martinez, L.O. Cota, S.D. Ferreira, G.L. Silva, J.E. Costa, Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J. Clin. Periodontol. 39(2), 173–181 (2012). https://doi.org/10.1111/j.1600-051X.2011.01819.x
M. Esposito, M.G. Grusovin, V. Loli, P. Coulthard, H.V. Worthington, Does antibiotic prophylaxis at implant placement decrease early implant failures? A Cochrane systematic review. Eur. J. Oral Implantol. 3(2), 101–110 (2010)
A. Tathe, M. Ghodke, A.P. Nikalje, A brief review: biomaterials and their application. Int. J. Pharm. Pharm. Sci. 2(4), 19–23 (2010)
S.R. Hanson, E.I. Tucker, R.A. Latour, Blood coagulation and blood–material interactions, in An Introduction to Materials in Medicine, Biomaterials Science, 4th edn. (Academic Press, 2020), pp. 801–812. https://doi.org/10.1016/B978-0-12-816137-1.00052-0
F. Albee, H. Morrison, Studies in bone growth: triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 71, 32–38 (1920). https://doi.org/10.1097/00000658-192001000-00006
W. Habraken, P. Habibovic, M. Epple, M. Bohner, Calcium phosphates in biomedical applications: materials for the future? Mater. Today 19(2), 69–87 (2016). https://doi.org/10.1016/j.mattod.2015.10.008
P.N. Kumta, C. Sfeir, D.H. Lee, D. Olton, D. Choi, Nanostructured calcium phosphates for biomedical applications: novel synthesis and characterization. Acta Biomater. 1, 65–83 (2005). https://doi.org/10.1016/j.actbio.2004.09.008
M.N. Hassan, M.M. Mahmoud, A. Abd El-Fattah, S. Kandil, Microwave-assisted preparation of nano-hydroxyapatite for bone substitutes. Ceram. Int. 42, 3725–3744 (2016). https://doi.org/10.1016/j.ceramint.2015.11.044
A. Nakahira, K. Nakata, C. Numako, H. Murata, K. Matsunaga, Synthesis and evaluation of calcium-deficient hydroxyapatite with SiO2. Mater. Sci. Appl. 2, 1194–1198 (2011). https://doi.org/10.4236/msa.2011.29161
X. Li, Y. Deng, M. Wang, X. Chen, Y. Xiao, X. Zhang, Stabilization of Ca-deficient hydroxyapatite in biphasic calcium phosphate ceramics by adding alginate to enhance their biological performances. J. Mater. Chem. B 6, 84–97 (2018). https://doi.org/10.1039/c7tb02620j
M. Bohner, B.G. Santoni, N. Dobelin, β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 113, 23–41 (2020). https://doi.org/10.1016/j.actbio.2020.06.022
R.G. Carrodeguas, S. De Aza, α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 7, 3536–3546 (2011). https://doi.org/10.1016/j.actbio.2011.06.019
J.C. Elliott, Structure and chemistry of the apatites and other calcium orthophosphates, ed. by H.M. Hughes, (Elsevier, Amsterdam, 1994)
M. Canillas, P. Pena, A.H. de Aza, M.A. Rodríguez, Calcium phosphates for biomedical applications. Boletín de la Sociedad Española de Cerámica Y vidrio 56, 91–112 (2017). https://doi.org/10.1016/j.bsecv.2017.05.001
N.A.S.M. Pu’ad, P. Koshy, H.Z. Abullah, M.I. Idris, T.C. Lee, Syntheses of hydroxyapatite from natural sources. Heliyon 5, 1–14 (2019). https://doi.org/10.1016/j.heliyon.2019.e01588
M.A. Taha, R.A. Youness, M.F. Zawrah, Review on nanocomposites fabricated by mechanical alloying. Int. J. Miner. Metall. Mater. 26(9), 1047–1058 (2019). https://doi.org/10.1007/s12613-019-1827-4
A. Szcześ, L. Holysz, E. Chibowski, Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 249, 321–330 (2017). https://doi.org/10.1016/j.cis.2017.04.007
R.A. Youness, M.A. Taha, H. Elhaes, M. Ibrahim, Molecular modeling, FTIR spectral characterization and mechanical properties of carbonated-hydroxyapatite prepared by mechanochemical synthesis. Mater. Chem. Phys. 190, 209–218 (2017). https://doi.org/10.1016/j.matchemphys.2017.01.004
R.A. Youness, M.A. Taha, H. Elhaes, M. Ibrahim, Preparation, Fourier transform infrared characterization and mechanical properties of hydroxyapatite nanopowders. J. Comput. Theor. Nanosci. 14, 2409–2415 (2017). https://doi.org/10.1166/jctn.2017.6841
R.A. Youness, M.A. Taha, M. Ibrahim, In vitro bioactivity, physical and mechanical properties of carbonated-fluoroapatite during mechanochemical synthesis. Ceram. Int. 44, 21323–21329 (2018). https://doi.org/10.1016/j.ceramint.2018.08.184
R.A. Youness, M.A. Taha, M. Ibrahim, Dense alumina-based carbonated fluorapatite nanobiocomposites for dental applications. Mater. Chem. Phys. 257, 123264 (2020). https://doi.org/10.1016/j.matchemphys.2020.123264
A. Refaat, R.A. Youness, M.A. Taha, M. Ibrahim, Effect of zinc oxide on the electronic properties of carbonated hydroxyapatite. J. Mol. Struct. 1147(5), 148–154 (2017). https://doi.org/10.1016/j.molstruc.2017.06.091
M. Sadat-Shojai, M.T. Khorasani, E. Dinpanah-Khoshdargi, A. Jamshidi, Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 9(8), 7591–7621 (2013). https://doi.org/10.1016/j.actbio.2013.04.012
J. Chen, Y. Wang, X. Chen, L. Ren, C. Lai, W. He, Q. Zhang, A simple sol-gel technique for synthesis of nanostructured hydroxyapatite, tricalcium phosphate and biphasic powders. Mater. Lett. 65, 1923–1926 (2011). https://doi.org/10.1016/j.matlet.2011.03.076
H. Eshtiagh-Hosseini, M.R. Housaindokht, M. Chahkandi, Effects of parameters of sol-gel process on the phase evolution of sol-gel derived hydroxyapatite. Mater. Chem. Phys. 106, 310–316 (2007). https://doi.org/10.1016/j.matchemphys.2007.06.002
A. Fihri, C. Len, R.S. Varma, A. Solhy, Hydroxyapatite: a review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 347, 48–76 (2017). https://doi.org/10.1016/j.ccr.2017.06.009
S.K. Swain, D. Sarkar, A comparative study: hydroxyapatite spherical nanopowders and elongated nanorods. Ceram. Int. 37, 2927–2930 (2011). https://doi.org/10.1016/j.ceramint.2011.03.077
D.S. Gomes, A.M.C. Santos, G.A. Neves, R.R. Menezes, A brief review on hydroxyapatite production and use in biomedicine. Ceramica 65, 869–872 (2019). https://doi.org/10.1590/0366-69132019653742706
M. Okada, T. Furuzono, Hydroxylapatite nanoparticles: fabrication methods and medical applications. Sci. Technol. Adv. Mater. 13, 1–14 (2012). https://doi.org/10.1088/1468-6996/13/6/064103
H.C. Shum, A. Bandyopadhyay, S. Bose, D.A. Weitz, Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite. Chem. Mater. 21, 5548–5555 (2009). https://doi.org/10.1021/cm9028935
S.K. Saha, A. Banerjee, S. Banerjee, S. Bose, Synthesis of nanocrystalline hydroxyapatite using surfactant template systems: role of templates in controlling morphology. Mater. Sci. Eng. C 29, 2294–2301 (2009). https://doi.org/10.1016/j.msec.2009.05.019
G. Guo, Y. Sun, Z. Wang, H. Guo, Preparation of hydroxyapatite nanoparticles by reverse microemulsion. Ceram. Int. 31, 869–872 (2005). https://doi.org/10.1016/j.ceramint.2004.10.003
M. Jamil, B. Elouatli, H. Khallok, A. Elouahli, E. Gourri, M. Ezzahmouly, F. Abida, Z. Hatim, Silicon substituted hydroxyapatite: preparation with solid-state reaction, characterization and dissolution properties. J. Mater. Environ. Sci. 9, 2322–2327 (2018)
G. Heinicke, Tribochemistry, ed. by Carl Hanser Verlag, (Munchen Publishers, 1984), p. 119
S. Adzila, I. Sopyan, M. Hamdi, Mechanochemical synthesis of hydroxyapatite nanopowders: effects of rotation speed and milling time on powder properties. AMM 110–116, 3639–3644 (2012). https://doi.org/10.4028/www.scientific.net/AMM.110-116.3639
R.A. Youness, M.A. Taha, M.A. Ibrahim, In vitro bioactivity, molecular structure and mechanical properties of zirconia-carbonated hydroxyapatite nanobiocomposites sintered at different temperatures. Mater. Chem. Phys. 239, 122011 (2020). https://doi.org/10.1016/j.matchemphys.2019.122011
P. Kamalanthan, R. Singh, L.T. Bang, A. Niakan, C.Y. Tan, J. Purbolaksono, H.C. Thambinayagam, W. Teng, Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 40(10B), 16349–16359 (2014). https://doi.org/10.1016/j.ceramint.2014.07.074
P.A.F. Sossa, B.S. Giraldo, B.C.G. Garcia, E.R. Parra, P.J.A. Arango, Comparative study between natural and synthetic hydroxyapatite: structural, morphological and bioactivity properties. Revista Materia 23(4), 12217 (2018). https://doi.org/10.1590/s1517-707620180004.0551
M.K. Herliansyah, D.A. Nasution, M. Hamdi, A. Ide-Ektessabi, M.W. Wildan, A.E. Tontowi, Preparation and characterization of natural hydroxyapatite: a comparative study of bovine bone hydroxyapatite and hydroxyapatite from calcite. Mater. Sci. Forum 561–565, 1441–1444 (2007). https://doi.org/10.4028/www.scientific.net/MSF.561-565.144
A. Ressler, K. Gudelj, M. Zadro, M. Antunović, M. Cvetnić, M. Ivanković, H. Ivanković, From bio-waste to bone substitute: synthesis of biomimetic hydroxyapatite and its use in chitosan-based composite scaffold preparation. Chem. Biochem. Eng. Q. 34(2), 59–71 (2020). https://doi.org/10.15255/CABEQ.2020.183
B.N. Alhussary, G.A. Taqa, A.A. Taqa, Preparation and characterization of natural nano hydroxyapatite from egg shell and seashell and its effect on bone healing. JAVS 5(2), 25–32 (2020). https://doi.org/10.21608/JAVS.2020.85567
L. Dou, Y. Zhang, H. Sun, Advances in synthesis and functional modification of nanohydroxyapatite. J. Nanomater. 2018, 1–7 (2018). https://doi.org/10.1155/2018/3106214
T. Laonapakul, Synthesis of hydroxyapatite from biogenic wastes. KKU Eng. J. 42(3), 269–275 (2015). https://doi.org/10.14456/kkuenj.2015.30
A.M. Torgalkar, A resonance frequency technique to determine elastic modulus of hydroxyapatite. J. Biomed. Mater. Res. 13(6), 907–920 (1979). https://doi.org/10.1002/jbm.820130609
P.N. De Aza, A.H. De Aza, S. De Aza, Crystalline bioceramic materials. Bol. Soc. Esp. Ceram. Vidr. 44(3), 135–145 (2005)
C.R. Bowen, J. Gittings, I.G. Turner, F. Baxter, J.B. Chaudhuri, Dielectric and piezoelectric properties of hydroxyapatite-BaTiO3 composites. Appl. Phys. Lett. 89, 1–3 (2006). https://doi.org/10.1063/1.2355458
M. Prakasam, M. Albino, E. Lebraud, M. Maglione, C. Elissalde, A. Largeteau, Hydroxyapatite-barium titanate piezocomposites with enhanced electrical properties. J. Am. Ceram. Soc. 100(6), 2621–2631 (2017)
S. Pokhrel, Hydroxyapatite: preparation, properties and its biomedical applications. Adv. Chem. Eng. Sci. 8, 225–240 (2018). https://doi.org/10.4236/aces.2018.84016
J.B. Foresman, Ab initio techniques in chemistry: interpretation and visualization, Chap. 14 in What Every Chemist Should Know About Computing, ed. M.L. Swift, T.J. Zielinski (ACS Books, Washington, D.C., 1996)
M. Ibrahim, A.A. Mahmoud, Computational notes on the reactivity of some functional groups. J. Comput. Theor. Nanosci. 6, 1523–1526 (2009). https://doi.org/10.1166/jctn.2009.1205
H.A. Ezzat, M.A. Hegazy, N.A. Nada, M.A. Ibrahim, Effect of nano metal oxides on the electronic properties of cellulose, chitosan and sodium alginate. Biointerface Res. Appl. Chem. 9(4), 4143–4149 (2019). https://doi.org/10.33263/BRIAC94.979986
A. Ibrahim, H. Elhaes, F. Meng, M. Ibrahim, Effect of hydration on the physical properties of glucose. Biointerface Res. Appl. Chem. 8(4), 4114–4118 (2019)
A.M. Bayoumy, H. Elhaes, O. Osman, K.T. Kholmurodov, T. Hussein, M.A. Ibrahim, Effect of nano metal oxides on heme molecule: molecular and bimolecular approaches. Biointerface Res. Appl. Chem. 10(1), 4837–4845 (2020). https://doi.org/10.33263/BRIAC101.837845
A.M. Bayoumy, H. Elhaes, O. Osman, T. Hussein, M.A. Ibrahim, Mapping molecular electrostatic potential for heme interacting with nano metal oxides. Biointerface Res. Appl. Chem. 10(2), 5091–5095 (2020)
G.W. Ali, W.I. Abdel-Fattah, H. Elhaes, M.A. Ibrahim, Spectroscopic and modeling analyses of bimolecular structure of corn silk. Biointerface Res. Appl. Chem. 9(6), 4481–4485 (2019). https://doi.org/10.33263/BRIAC0102.091095
M.M. El-Sayed, A. Omar, M. Ibrahim, W.I. Abdel-Fattah, On the structural analysis and electronic properties of chitosan/hydroxyapatite interaction. J. Comput. Theor. Nanosci. 6, 1663–1669 (2009). https://doi.org/10.1166/jctn.2010.1363
A. Wierzbicki, H.S. Cheung, Molecular modeling of inhibition of hydroxyapatite by phosphocitrate. J. Mol. Struct. THEOCHEM 529(1–3), 73–82 (2000). https://doi.org/10.1016/S0166-1280(00)00534-0
J. Zhao, L. Wu, C. Zhan, Q. Shao, Z. Guo, L. Zhang, Overview of polymer nanocomposites: computer simulation understanding of physical properties. Polymer 133, 272–2872 (2017). https://doi.org/10.1016/.polymer.2017.10.035
N. Zhang, Y. Cheng, X. Hu, J. Yeo, Toward rational algorithmic design of collagen-based biomaterials through multiscale computational modeling. Curr. Opin. Chem. Eng. 24, 79–87 (2019). https://doi.org/10.1016/j.coche.2019.02.011
M. Nouri-Felekori, M. Khakbiz, N. Nezafati, J. Mohammadi, M.B. Eslaminejad, N. Fani, Characterization and multiscale modeling of novel calcium phosphate composites containing hydroxyapatite whiskers and gelatin microspheres. J. Alloys Compd. 832, 154938 (2020). https://doi.org/10.1016/j.jallcom.2020.154938
G.E. Dubinenko, A.L. Zinoviev, E.N. Bolbasov, V.T. Novikov, S.I. Tverdokhlebov, Preparation of poly(L-lactic acid)/hydroxyapatite composite scaffolds by fused deposit modeling 3D printing. Mater. Today Proc. 22(2), 228–234 (2020). https://doi.org/10.1016/j.matpr.2019.08.092
C.M. Garcia, S.A. Toms, A cautionary tale of hydroxyapatite cement use in frontal sinus obliteration. Interdiscip. Neurosurg. 21, 100702 (2020). https://doi.org/10.1016/j.inat.2020.100702
S. Bose, S. Tarafder, Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 8(4), 1401–1421 (2012). https://doi.org/10.1016/j.actbio.2011.11.017
U. Gbureck et al., Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv. Funct. Mater. 17(18), 3940–3945 (2007). https://doi.org/10.1002/adfm.200700019
T. Tian, C. Wu, J. Chang, Preparation and in vitro osteogenic, angiogenic and antibacterial properties of cuprorivaite (CaCuSi4O10, Cup) bioceramics. RSC Adv. 6(51), 45840–45849 (2016). https://doi.org/10.1039/C6RA08145B
X. Lin, S. Patil, Y.-G. Gao, A. Qian, The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 11, 1–15 (2020). https://doi.org/10.3389/2fphar.2020.00757
M.-Y. Shie, S.-J. Ding, H.-C. Chang, The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 7(6), 2604–2614 (2011). https://doi.org/10.1016/j.actbio.2011.02.023
R.A. Youness, M.A. Taha, M.A. Ibrahim, Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 1150, 188–195 (2017). https://doi.org/10.1016/j.molstruc.2017.08.070
R.A. Youness, M.A. Taha, A.A. El-Kheshen, M. Ibrahim, Influence of the addition of carbonated hydroxyapatite and selenium dioxide on mechanical properties and in vitro bioactivity of borosilicate inert glass. Ceram. Int. 44, 20677–20685 (2018). https://doi.org/10.1016/j.ceramint.2018.08.061
M.A. Taha, R.A. Youness, M. Ibrahim, Biocompatibility, physico-chemical and mechanical properties of hydroxyapatite-based silicon dioxide nanocomposites for biomedical applications. Ceram. Int. 46, 23599–23610 (2020). https://doi.org/10.1016/j.ceramint.2020.06.132
D. Arcos, M. Vallet-Regí, Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 8, 1781–1800 (2020). https://doi.org/10.1039/c9tb02710f
R. Chaharmahali, A. Fattah-Alhosseini, H. Esfahani, Increasing the in-vitro corrosion resistance of AZ31B-Mg alloy via coating with hydroxyapatite using plasma electrolytic oxidation. J. Asian Ceram. Soc. 8, 39–49 (2020). https://doi.org/10.1080/21870764.2019.1698143
T.J. Levingstone, S. Herbaj, J. Redmond, H.O. McCarthy, N.J. Dunne, Calcium phosphate nanoparticles-based systems for RNAi delivery: applications in bone tissue engineering. Nanomaterials 146(10), 1–28 (2020). https://doi.org/10.3390/nano10010146
X. Zeng, H. Xu, J. Lu, Q. Chen, W. Li, L. Wu, J. Tang, L. Ma, The immobilization of soil cadmium by the combined amendment of bacteria and hydroxyapatite. Sci. Rep. 10, 2198 (2020). https://doi.org/10.1038/s41598-020-58259-1
J.A.G. del Rio, P.J. Morando, D.S. Cicerone, Natural materials for treatment of industrial effluents: comparative study of the retention of Cd, Zn and Co by calcite and hydroxyapatite. Part I: batch experiments. J. Environ. Manage. 71, 169–177 (2004). https://doi.org/10.1016/j.jenvman.2004.02.004
Ramdani, A. Kadeche, M. Adjdir, Z. Taleb, D. Ikhou, S. Taleb, A. Deratani, Lead and cadmium removal by adsorption process using hydroxyapatite porous materials, Water Pract. Technol. 15(1), 130–141 (2020). https://doi.org/10.2166/wpt.2020.003
K. Usami, A. Okamoto, Hydroxyapatite: catalyst for a one-pot pentose formation. Org. Biomol. Chem. 15, 8888–8893 (2017). https://doi.org/10.1039/c7ob02051a
S. ben Moussa, A. Mehri, B. Badraoui, Magnesium modified calcium hydroxyapatite: an efficient and recyclable catalyst for the one-pot Biginelli condensation. J. Mol. Struct. 1200, 127111 (2020). https://doi.org/10.1016/j.molstruc.2019.127111
D. Milovac, I. Weigand, M. Kovacic, M. Ivankovic, H. Ivankovic, Highly porous hydroxyapatite derived from cuttlefish bone as TiO2 catalyst support. Process. Appl. Ceram. 12(2), 136–142 (2018). https://doi.org/10.2298/PAC1802136M
S.C. Oh, J. Xu, D.T. Tran, B. Liu, D. Liu, Effects of controlled crystalline surface of hydroxyapatite on methane oxidation reactions. ACS Catal. 8(5), 4493–4507 (2018). https://doi.org/10.1021/acscatal.7b04011
M. Shokouhimehr, S.M.G. Yek, M. Nasrollahzadeh, A. Kim, R.S. Varma, Palladium nanocatalysts on hydroxyapatite: green oxidation of alcohol and reduction of nitroarenes in water. Appl. Sci. 9, 1–12 (2019). https://doi.org/10.3390/app9194183
J. Xu, T. White, P. Li, C. He, Y.F. Han, Hydroxyapatite foam as a catalyst for formaldehyde combustion at room temperature. J. Am. Chem. Soc. 132(38), 13172–13173 (2010). https://doi.org/10.1021/ja1058923
M.B. Taşkın, Ö. Şahin, H. Taskin, O. Atakol, A. Inal, A. Gunes, Effect of synthetic nano-hydroxyapatite as an alternative phosphorus source on growth and phosphorus nutrition of lettuce (Lactuca sativa L.) plant, J. Plant Nutr. 41(9), 1148–1154 (2018). https://doi.org/10.1080/01904167.2018.1433836
N. Kottegoda, C. Sandaruwan, G. Priyadarshana, A. Siriwardhana, U.A. Rathnayake, D.M.B. Arachchige, A.R. Kumarasinghe, D. Dahanayake, V. Karunaratne, G.A. Amaratunga, Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 11(2), 1214–1221 (2017). https://doi.org/10.1021/acsnano.6b07781
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ibrahim, M., Youness, R.A., Taha, M.A. (2024). Overview of Some Production Routes for Hydroxyapatite and Its Applications. In: Ikhmayies, S.J. (eds) Advances in Minerals Research. Advances in Material Research and Technology. Springer, Cham. https://doi.org/10.1007/978-3-031-49175-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-49175-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-49174-0
Online ISBN: 978-3-031-49175-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)