Abstract
Crystalloids are commonly used in medicine as solutions containing electrolytes dissolved in water, with or without glucose. They can be used as maintenance, replacement, or resuscitation fluids, but should be administered with caution. This chapter provides an overview of basic definitions, terminology, and concepts regarding crystalloids, including their categorization by tonicity, their balanced or unbalanced nature, and the importance of strong ion difference (SID). Improper administration of crystalloids can lead to morbidity, particularly hyperchloremic metabolic acidosis (HMA) and fluid overload. Moreover, saline with a SID of zero can cause a positive sodium balance and subsequent fluid accumulation, which can lead to renal dysfunction and the need for vasopressors and renal replacement therapy. Recent systematic reviews and post-hoc analyses of six major fluid trials have shown that balanced solutions (not containing glucose) reduce mortality by 1%, making them a good first choice for resuscitation in patients with sepsis and septic shock, burns, or diabetic ketoacidosis. Traumatic brain injury and gastrointestinal losses may be the only indications left for (ab)normal saline. The pediatric community still favors isotonic solutions for maintenance, although a growing body of evidence supports hypotonic crystalloids as a better choice. Hypertonic crystalloids have been described for small volume resuscitation in specific patient populations, such as post cardiac arrest, but their sodium burden may outweigh the temporarily beneficial hemodynamic effects. In case of excessive losses, fluids should be substituted or replaced by those that mimic the fluids that are lost, such as blood. Prescribing crystalloid solutions should be done with care. Fluid overload or accumulation and HMA should be avoided, as it can induce extra morbidity and mortality. Choosing the right fluid, indication, dose, and duration is crucial for preventing morbidity and mortality: it is all about giving the right dose of the right fluid at the right time for the right patient!
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
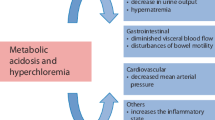
FormalPara IFA Commentary (MLNGM)This chapter takes you back to the basics with an overview of basic definitions, terminology, and concepts. Crystalloids are solutions that contain electrolytes dissolved in water and other small water-soluble molecules, with or without dextrose or glucose. They are widely used as maintenance solutions, replacement solutions, or resuscitation fluids. Crystalloids are categorized by their tonicity relative to plasma and can be isotonic, hypotonic, or hypertonic. And they can be balanced (or buffered) with a strong ion difference (SID) close to plasma or unbalanced (like NaCl 0.9% with a SID of zero). The SID is important for the effect on the acid–base status after administration. There is more and more evidence that imprudent administration of crystalloids may lead to morbidity. There are two major concerns in administering crystalloids: First is the induction of hyperchloremic metabolic acidosis (HMA), a proven side effect of saline. Although animal studies showed HMA can lead to kidney dysfunction and it also seemed to induce morbidity in normal volunteers, there was little data on relevant clinical parameters. There is also rising evidence that saline can lead to a delay in micturition, although the exact mechanism is unclear. Second is the induction of fluid overload or accumulation. It is frequently shown that more crystalloids than colloids are needed to achieve clinical stability. Historically, colloid vs crystalloid studies showed conflicting data in this matter but in critically ill shocked patients the volume expansion effects of crystalloids and colloids may be similar based on their pharmacokinetic and dynamic properties. The induction of a positive sodium balance also accompanied with fluid accumulation is another explanation why saline may induce fluid accumulation. Even normal kidneys may take days if not weeks to get rid of the excess sodium. Other deleterious effects of saline are increased potassium levels, renal hypoperfusion, and increased need for vasopressors and renal replacement therapy. A recent systematic reviews and post-hoc analyses of the latest major fluid trials including almost 35,000 ICU patients have shown a 90% probability that balanced solutions reduce mortality by 1% (range −9 to +1%). Figure 9.1 shows the combined summary of findings.
Therefore, in patients with sepsis and septic shock, burns, or diabetic ketoacidosis, balanced or buffered crystalloids (not containing glucose) are a good first choice but not in patients with traumatic brain injury where saline is preferred. Gastrointestinal losses may be another indication for (ab)normal saline as it may help to correct hypochloremic metabolic alkalosis caused by losses. There is also growing body of evidence that maintenance solutions should be hypotonic crystalloids, although the pediatric community still favors isotonic solutions. Hypertonic crystalloids have been described for small volume resuscitation in specific patient populations (e.g., post cardiac arrest) but the sodium burden may outweigh the temporarily beneficial hemodynamic effects. In case of excessive losses, fluids should be substituted or replaced by those, mimicking the fluids that are lost (e.g., blood). Crystalloid solutions should be prescribed with the same care and caution as we do with medication, by giving the right dose of the right fluid at the right time. When using crystalloids, avoiding HMA by using balanced solutions seems to be important, although the critical dose for a switch from saline is not known. Fluid accumulation is to be avoided as it is proven to induce morbidity and mortality.
FormalPara Learning ObjectivesAfter reading this chapter, you will understand that:
-
1.
Half-life of intravenous fluids is dependent on the pharmacokinetics and pharmacodynamic properties of the specific fluid.
-
2.
Nearly 75% of isotonic crystalloid fluids leaves intravascular space to interstitial after administration.
-
3.
A 0.9% saline is not “normal” and can cause dilutional hyperchloremic metabolic acidosis, renal and splanchnic vasoconstriction, glycocalyx, and coagulation dysfunction, especially, when administered in large volumes.
-
4.
The evidence on the benefit of balanced crystalloids over 0.9% saline is equivocal. Because of the physiological rationale of balanced salt solutions and the risk of harm associated with 0.9% saline, they are the resuscitation fluids of choice for most patients with sepsis, burns, or diabetic ketoacidosis.
-
5.
Saline is preferred over balanced solutions in patients with traumatic brain injury and gastrointestinal losses.
-
6.
Hypertonic saline may be used for small volume resuscitation or the treatment of raised intracranial pressure or severe symptomatic hyponatremia. However, frequent monitoring of serum sodium and osmolality is recommended with serum sodium not exceeding 12 mEq over 24 h and 18 mEq over 48 h.
-
7.
Sodium bicarbonate administration may cause paradoxical acidosis with intracellular acidosis, and there is lack of evidence supporting the use of sodium bicarbonate for correction of metabolic acidosis on any patient-centered outcomes.
Mr. B, an 82-year-old male, with past history of hypertension on hydrochlorothiazide, was admitted with history of acute central abdominal pain for the past few days, associated with vomiting. On examination, he was drowsy but obeying simple commands, his extremities were cool to touch with a heart rate of 108/min, he has a blood pressure of 70 mmHg systolic, respiratory rate 28/min, and temperature 36.9 °C. His abdomen was distended and diffusely tender with absent bowel sound. Arterial blood gas analysis showed evidence of high anion gap metabolic acidosis with lactate 5 mmol/L. Combined with the CT findings of a pneumoperitoneum, a diagnosis of bowel perforation with peritonitis and septic shock was made. He was planned for an emergency laparotomy after initial resuscitation. At laparotomy, he was found to have a duodenal perforation with bowel loop adhered to it. Perforation repair and peritoneal toileting was performed, and he was moved to the ICU.
Questions
-
Q1. What will be the most appropriate fluid for initial resuscitation of this patient?
-
Q2. Which fluid to be chosen for maintenance intravenous therapy now?
Introduction
Over the centuries, intravenous fluid therapy has become an integral part of therapeutic intervention in critically sick patients. Crystalloids and colloids have been the mainstay of intravenous fluid resuscitation. First successful use of a crystalloid solution was by Thomas Latta in 1832, who infused a solution of saline and sodium bicarbonate in cholera patients [1]. In 1876, Sidney Ringer developed a fluid comparable to blood plasma that enabled a frog’s heart to continue beating in vitro [2]. In 1932, Alex Hartmann modified Ringer’s solution by adding lactate as a buffer and used it to rehydrate children suffering from gastroenteritis [3].
Crystalloids are described as fluids containing electrolytes (e.g., sodium, potassium, chloride). They lack the large proteins and molecules found in colloids and plasma, and 0.9% saline has been the most commonly prescribed crystalloid over many years. But recently, balanced solutions are catching more attention. Despite the ubiquity of fluid therapy, this intervention remains a subject of an ongoing controversy. An “ideal fluid” remains elusive. More information on albumin use can be found in Chap. 10, while other colloid solutions like starches and gelatins are discussed in Chap. 11.
Fluid Physiology
All intravascular fluids tend to redistribute throughout the body. After administration, intravascular half-life of any given intravenous fluid varies depending on the pharmacokinetic and pharmacodynamic properties of the fluid. Some of the factors that determine the in vivo activity of intravenous fluids are its tonicity, oncotic pressure, acid–base properties, and integrity of the endothelial glycocalyx (see Chaps. 2 and 3). A fundamental rationale for intravascular fluid resuscitation is to sustain an effective circulating intravascular volume. Interestingly, around 75% of a crystalloid volume load ends up in the interstitium.
Total body water (TBW) is divided functionally into extracellular (ECW) and the intracellular water (ICW), confined to dedicated fluid spaces separated by the cell membrane. ECW is further divided into intravascular and interstitial fluid spaces (Table 9.1). Figure 9.2 illustrates fluid composition in a 70 kg male. These two compartments of ECW are separated by capillary membrane with pores. Intravascular volume depends on the net balance between plasma oncotic pressure and hydrostatic pressures. This relationship was mathematically expressed by Starling in his famous Starling equation [4]:
Pc hydrostatic pressure in the capillary, Pi hydrostatic pressure in the interstitium, pc oncotic pressure in the capillary, pi oncotic pressure in the interstitium.
Fluid distribution in a 70 kg man. The human body consists of 60% water. The total body water (TBW) is separated into intracellular water (ICW, 66%) and extracellular water (ECW, 33%). The ECW consists of the intravascular fluid (IVF, 25%) and extravascular fluid (EVF, 75%), mainly interstitial fluid
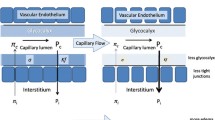
More recently, Starling’s description of fluid dynamics has been challenged. With the discovery of endothelial glycocalyx, a lining inside the endothelium, it is now realized that movement of fluid is much more complex. Glycocalyx is negatively charged and contributes as a natural barrier of the vessel walls. Glycocalyx is fragile and is affected by various factors like ischemia, sepsis, hypoxia, and inflammation. Woodcock and colleagues proposed a revised Starling model that considers the composition of intravascular fluid, interstitial fluid, and physical characteristics of the transvascular barrier, i.e., endothelial glycocalyx [5]. This revised model shows that at low capillary hydrostatic pressures, transcapillary fluid losses for both crystalloids and colloids are similar [5]. Starling’s model and its revised form are described in more detail in Chap. 2.
It is vital to understand the mechanism for acid–base disturbances in critically ill patients which is important for the appropriate prescription of intravenous fluid (traditional acid–base concepts are discussed in Chap. 6). Stewart’s quantitative physical chemical approach enables us to understand the acid–base properties of intravenous fluids. Stewart’s concept is described in Chap. 6.
Types of Crystalloids
Crystalloids have been classified on the basis of their tonicity (compared to that of plasma), their effects on acid–base balance, and their clinical use.
Tonicity
Tonicity or “effective osmolality” is an important property of body fluids, as it determines movement of water between extracellular and intracellular compartments (see Chap. 2). Osmolality of a solution is defined as the amount of solute (in mmol/L) dissolved in the solvent (e.g., water) measured in kilogram (kg). Normal plasma tonicity is 270–290 mOsm/kg.
Another closely related term is osmolarity, defined as the amount of solute dissolved in a solution measured in liter (mOsm/L).
Isotonic Crystalloids
Isotonic crystalloids have a tonicity close to plasma. When administered to a normally hydrated patient, isotonic crystalloids do not cause a significant shift of water between the blood vessels and the cells. Thus, there is no (or minimal) osmosis occurring. Commonly prescribed isotonic fluids are Ringer’s lactate (Hartmann’s), Ringer’s acetate, Plasma-Lyte, or dextrose 5% in saline 0.9%.
Hypertonic Crystalloids
Hypertonic crystalloids have a tonicity higher than plasma. Administration of a hypertonic crystalloids causes water to shift from the extravascular space into the intravascular space thereby increasing the intravascular volume. This osmotic shift occurs as the body attempts to dilute the higher concentration of electrolytes contained within the hypertonic fluid by moving water into the intravascular space. Hypertonic solutions may result in cellular dehydration. A commonly used hypertonic crystalloid is 3% saline. Other concentrations are 5%, 7.5%, and 23% saline.
Hypotonic Crystalloids
Hypotonic crystalloids have a tonicity lower than plasma. Administration of a hypotonic crystalloid causes water to shift from the intravascular space to the extravascular space because of the higher concentration of electrolytes in the extravascular spaces. This shift of fluid eventually transmits into the tissue cells. Hypotonic solutions may result in cellular hydration. Commonly used hypotonic solutions are 5% dextrose, 10% dextrose, and dextrose in hypotonic saline (5% dextrose + 0.45% saline).
Unbalanced Crystalloids
Unbalanced crystalloids have been described as intravenous crystalloid solutions having a high chloride concentration in comparison with plasma chloride levels (96–106 mEq/L). Examples of unbalanced crystalloids are 0.9% saline, 3% saline, etc.
Balanced Crystalloids
Balanced (or buffered) crystalloids are defined as intravenous crystalloid solutions whose electrolyte composition is closer to that of plasma with a strong ion deficit (SID) around 24 mmol/L. They contain physiological or near physiological amounts of chloride. The commonest balanced fluids are Ringer’s lactate, Ringer’s acetate, Plasma-Lyte, and Sterofundin.
Isotonic Crystalloids
Crystalloids have been widely used in resuscitating patients with dehydration, trauma, burn, and other shock states including septic shock. They along with colloids have been the mainstay of resuscitation though the latter has fallen out of favor because of their deleterious effects on the human body (discussed in more detail in Chaps. 10 and 11). Major guidelines including surviving sepsis guidelines recommend isotonic crystalloid as the initial choice of fluid for resuscitation [6, 7]. Isotonic crystalloids are also routinely used as maintenance fluids especially in children. In this section, we shall discuss about isotonic crystalloids most widely used for resuscitation (Table 9.2).
Isotonic Saline or 0.9% Saline
Normal saline is a 0.9% preparation of sodium chloride, equivalent to 154 mmol/L of sodium (Na) and chloride (Cl). If sodium chloride completely dissociated in solution, the expected osmolality would be two times 154, or 308 mOsm/kg. Interestingly, in-vivo measured effective osmolality (tonicity) of 0.9% saline of 286 mOsmol/L makes it isotonic to plasma, because a small percentage remains non-ionized in water. As such, this fits nicely in the normal range of blood osmolality, of 275–290 mOsm/L.
The term “normal” is often misunderstood. Normal solution in physicochemistry is described as a solution where 1 mol, or 1 g weight equivalent, of the salt is dissolved in 1 kg of water. This is not the case with 0.9% saline, which derived its name from red-cell lysis studies performed in the 1880s which suggested that the concentration of salt in the blood is 0.9%; hence, it is “normal” ECF. However, this seems not correct, but is beyond the scope of this chapter.
The “isotonic,” “0.9% saline,” or normal saline was developed by Dr. Hartog Jacob Hamburger. It remains unknown how 0.9% saline became known as “normal.” Despite being described as normal or physiological, 0.9% saline differs significantly from plasma including much higher chloride content, SID of 0, and absence of electrolytes except Na and Cl (Table 9.2).
A major issue associated with 0.9% saline is dilutional hyperchloremic metabolic acidosis, seen with infusion of large volumes of saline. Using the term “dilutional hyperchloremic metabolic acidosis” instead of hyperchloremic acidosis is more appropriate as it considers SID changes as well as variations in volume and chloride concentration.
Biological effects of 0.9% saline have been shown by numerous studies. In a study of patients awaiting intra-abdominal surgery, SID decreased from 40 to 31 mEq/l with a simultaneous increase in chloride from 105 to 115 mEq/l, following infusion of 6 l of 0.9% saline over 2 h [8].
Animal studies and some clinical data suggest hyperchloremic metabolic acidosis as a proinflammatory stimulus causing renal and splanchnic vasoconstriction and circulatory and coagulation dysfunction. Renal effects of dilutional hyperchloremic acidosis are most widely described. In a study in human volunteers, Chowdhury and colleagues demonstrated a decrease in renal blood flow and renal cortical perfusion following infusion of 0.9% saline compared to Plasma-Lyte [9]. However, these changes in renal blood flow and renal perfusion following 0.9% saline infusion were not associated with increased concentration of urinary neutrophil gelatinase-associated lipocalin (NGAL), an early marker of kidney injury. The decrease in renal blood flow is possibly related to high chloride content in the distal convoluted tubule following 0.9% saline and tubuloglomerular feedback. In a recent review, Lobo and Awad listed a number of adverse consequences of administering 0.9% saline (Table 9.3) [11]. However, some of these adverse effects may be manifested only at a very high dose and many of these effects are not seen in clinical studies.
The primary advantage of 0.9% saline, over balanced crystalloids, is cost, as it is significantly cheaper.
Balanced Crystalloids
Balanced (or buffered) solutions contain different organic anions (such as lactate, acetate, malate, pyruvate, and gluconate) to maintain the electrical neutrality. Metabolization of these organic anions increases the SID of these solutions in vivo. Hence, these solutions become hypotonic in vivo. Despite having an electrolyte content closer to plasma, balanced solutions are neither perfect nor physiological. The concentrations of these organic anions present in these solutions are much higher than those of plasma. For example, lactate content of Ringer’s lactate is >25 times than that of plasma. These organic anions have variable effects in vivo. Compared to lactate, acetate has less effect on oxygen consumption and carbon dioxide elimination, and it is also metabolized by extrahepatic tissues. But high levels of acetate may lead to hypotension and myocardial toxicity. Gluconate is metabolized more slowly than lactate. Interestingly, plasma gluconate elevations following Plasma-Lyte infusion can cause false-positive tests for galactomannan (a marker used for early detection of systemic mycoses especially aspergillosis). Effects of organic anion (or buffering substances) are discussed in greater detail in Chap. 24. The SID of balanced crystalloids is different (29 mEq/l for Ringer’s lactate compared to 50 mEq/l for Plasma-Lyte) producing variable effect on acid–base balance (discussed in Chaps. 6 and 7).
Traditionally, it is believed that balanced crystalloids are contraindicated in the presence of hyperkalemia or in patients at a higher risk of hyperkalemia (e.g., chronic kidney disease) because of their K+ content. However, multiple studies have failed to confirm this concept. In a randomized controlled trial (RCT), O’Malley and colleagues compared the effects of 0.9% saline vs Ringer’s lactate for intraoperative intravenous fluid therapy in kidney transplant patients [10]. The study was prematurely terminated after enrolling 51 patients as a significantly higher number of patients in saline group developed hyperkalemia (defined as serum K+ >6 mmol/L) requiring anti-hyperkalemic measures. There are two possible explanations of balanced fluid not producing hyperkalemia. First, K+ content of balanced crystalloids gets rapidly diluted in the large extracellular fluid compartment. Second, contrary to 0.9% saline, balanced crystalloids do not produce dilutional hyperchloremic metabolic acidosis and mobilize K+ from intracellular compartment.
Another possible issue is related to co-administration of balanced crystalloids with blood transfusion, because of the theoretical concern about calcium salt being present in certain balanced fluids (e.g., Ringer’s lactate or Sterofundin) and possible precipitation of citrate and clot formation. Again, this has not been proven in clinical studies [12]. Plasma-Lyte is approved by the U.S. FDA as suitable for use with blood products.
Buffering substances in the balanced solutions (lactate, acetate, maleate, and gluconate) are metabolized primarily in the liver, and compromised liver function may affect the metabolism of these substances. The metabolism of lactate is affected most, compared to acetate (as acetate is metabolized in other organs too). In a rat model of hemorrhagic shock, Egin and colleagues tested Ringer’s lactate, Ringer’s acetate, Plasma-Lyte, and 0.9% saline in the presence or absence of a 70% partial liver resection [13]. The authors concluded that 0.9% saline is the most inappropriate fluid for resuscitation during shock in the presence of hepatic failure. Buffering capacity of lactate is overwhelmed by hepatic failure, whereas acetate metabolism remains uncompromised. Gluconate is excreted largely unchanged in urine, not being affected by hepatic dysfunction and not having much buffering effects. Differential effects of different buffers are discussed further in Chap. 23.
Clinical Evidence: 0.9% Saline Vs Balanced
Observational Studies
In a before-and-after single center study, Yunos and colleagues tested the effect of restricting chloride-rich fluid on renal outcome and mortality. They collected baseline data for 6 months when the ICU was predominantly using chloride-rich fluids (0.9% saline, succinylated gelatin, and 5% albumin) followed by a phaseout period of 6 months before switching to chloride-restricted fluid strategy (Plasma-Lyte, hyperoncotic albumin) [14]. Rise in creatinine, incidence of new-onset acute kidney injury (AKI) with RIFLE I and F class, and need for renal replacement therapy (RRT) were significantly reduced during the chloride restriction period. However, there was no difference in mortality or hospital/ICU length of stay between two periods. In a subsequent study, Yunos and colleagues extended the chloride restriction strategy for another 6 months and also collected retrospective data for chloride liberal period for additional 6 months [15]. The chloride-restricted strategy continued to be associated with decreased incidence of AKI and need for RRT. But interestingly, the incidence of AKI in the extended chloride-restricted period was higher compared to the original observation period! Both of Yunos’ studies were criticized for following reasons—open-label design, change in the bundle of care, not a single intervention, and possible Hawthorne effect. In a large retrospective observational study, Raghunathan and colleagues evaluated the effect of 0.9% saline vs some balanced crystalloids as resuscitation fluid in the first 2 days [16]. The balanced crystalloids group had lower mortality, and mortality was further reduced in patients receiving higher percentage of balanced crystalloids.
Randomized Controlled Studies
In the SPLIT study, a double-blind, double-crossover, cluster RCT conducted in four ICUs, patients requiring intravenous crystalloids were randomized to receive either 0.9% saline or Plasma-Lyte [17]. The incidence of AKI at 90 days, the primary outcome of the study, was not different between two groups. There was no difference in 90-day mortality, need for RRT, or other secondary outcomes between the groups. However, the study was criticized because of following reasons: First, indications for crystalloid use (resuscitation, maintenance, or replacement) were not specified. Second, mostly postoperative patients were enrolled. Third patients enrolled were not so sick (median APACHE II score ~14, 4% patients with sepsis or 2.5% with traumatic brain injury). Fourth, chloride levels were not measured. Finally, median volume of fluid received was only 2000 ml [17].
The SALT-ED study was a single-center, unblinded, multiple-crossover trial comparing balanced crystalloids (Ringer’s lactate or Plasma-Lyte) vs 0.9% saline among adults treated with intravenous fluid in the emergency department (ED) and were admitted to the hospital outside the ICU [18]. A total of 13,347 patients were enrolled, with a median crystalloid volume administered in ED of ~1000 ml. There was no difference in the number of hospital free days, the primary outcome of the study, between two groups. However, the incidence of major adverse kidney events (a combination of death, persistent AKI at day 30, or new need of RRT, MAKE30), a secondary outcome, was significantly lower in the balanced crystalloids group (4.7% vs 5.6%, P = 0.01). It was primarily driven by the lower incidence of AKI (defined as doubling of creatinine), not mortality nor the need of RRT [18].
In the SMART study, more than 15,000 patients admitted in five ICUs of a university hospital were randomized to receive either 0.9% saline or balanced crystalloids (Ringer’s lactate or Plasma-Lyte) as intravenous fluid [19]. The primary outcome, MAKE30, was significantly lower in the balanced crystalloid group (14.3% vs 15.4%, p = 0.04). There was also a trend towards higher 30-day mortality in the 0.9% saline group. However, the individual components of MAKE30, mortality before discharge or at day 30, need for new RRT, and persistent kidney dysfunction at 30 days were not different between two groups. There were important limitations of both (SMART and SALT-ED) studies: First, both were single-center, nonblinded studies requiring external validation of the data. Second, patient populations were not so sick with low overall mortality. Third, balanced crystalloids with different compositions (Ringer’s lactate and Plasma-Lyte) were clubbed together. Fourth, overall fluid volume received were low (median volume received in SMART ~1000 ml; median volume ED admission to wards in SALT-ED ~1000 ml). Finally, composite outcome of MAKE30 with giving similar weightage to death, RRT, and persistent renal dysfunction to decide MAKE30 may not be a true patient centered outcome [19].
Afterwards, the BASICS trial randomized 11,052 patients from 75 Brazil ICUs. They performed a factorial 2 × 2 randomization in 1:1:1:1 ratio to each fluid (balanced solution: Plasma-Lyte and 0.9% sodium chloride) and each rate of administration (333 ml/h and 999 ml/h). The conclusions were that the use of a balanced crystalloid compared to 0.9% sodium chloride did not reduce 90-day mortality [20] nor did the use of slower infusion rates, when a fluid bolus is required compared to a faster rate of infusion [21]. A post-hoc analysis showed that there is a high probability that balanced solution use in the ICU reduces 90-day mortality in patients who exclusively received balanced fl-uids before trial enrollment [22]. Another post-hoc analysis showed that among patients with sepsis, the effect of balanced crystalloids vs 0.9% saline on mortality was greater for those whom fluid choice was controlled starting in the ED compared with starting in the ICU [23].
Finally, another recent RCT (the PLUS study) compared Plasma-Lyte 148 to 0.9% saline, involving 5,037 patients from 53 ICUs of Australia and New Zealand. No increased risk of the 90 days mortality was observed with 0.9% saline (22% vs 21.8%, p = 0.9) compared to Plasma-Lyte 148. There was also no significant increased incidence of AKI (mean maximal risk in creatinine of 0.41 ± 1.02 mg/dl vs 0.41 ± 1.06 mg/dl) or need of RRT (12.9% vs 12.7%) with the use of 0.9% saline compared to Plasma-Lyte 148. The study was prematurely terminated due to disruptions from the COVID-19 pandemic. However, the futility cutoff was achieved before the termination and it was unlikely that results would have been different, if the trial continued. There were a large number of protocol deviations, with the use of nonstudy fluids in both groups. Finally, fluids used outside ICUs were not controlled and recorded [24].
Subsequently, researchers from these RCTs performed a metanalysis including13 RCTs and 35,884 patients. From the six RCTs with a low risk of bias (34,450 patients), including the PLUS study, the use of balanced crystalloids compared to 0.9% saline in critically ill patients was found to produce 9% relative reduction in mortality to 1% relative increase in mortality. There was high probability of 90% of reduction of mortality with the balanced crystalloids. In patients with sepsis, the effect was further pronounced with a range of 14% relative reduction of mortality to 1% increase [25].
To conclude, the negative effects of dilutional hyperchloremic acidosis on patient-centered outcome have not yet been documented unequivocally. But from a physiological standpoint and from limited evidence available so far, balanced fluids are superior over saline especially when administered in larger volumes during resuscitation. The use of 0.9% saline may still have a limited role in resuscitating patients with possible raised intracranial pressure, in replacing gastric fluid loss, as drug diluent (when dextrose 5% is contraindicated), or when no other isotonic crystalloid is available for resuscitation.
Hypotonic Crystalloids
Hypotonic fluids have tonicity lower than plasma and the osmolality varies, depending on its constituents. The addition of dextrose to hypotonic fluids helps to create isosmotic environment to prevent intravascular hemolysis with their administration. However, with the intracellular movement or metabolism of dextrose, the fluid becomes hypotonic. They are freely redistributed to the interstitium and intracellular compartment based on total body water composition, i.e., nearly two-third of the infused volume will move into the intracellular space.
Hypotonic crystalloid solutions are mainly used as maintenance fluids. Other use includes the treatment of hypernatremia with solute-free water deficits and drug diluents. The maintenance fluids are required to replace sensible (e.g., urine, feces, sweat) and insensible (e.g., cutaneous or respiratory evaporative losses, fever) losses, in those who are unable to replace them enterally. The best maintenance fluid is the one that has not been administered. One should only start maintenance solutions if the patient is not able to cover his/her daily fluid needs (25 ml/kg/day) orally or enterally.
Solute-free water is lost with insensible losses, and therefore more water than solutes are needed for maintaining fluid balance. The sodium concentration in these fluids is between 40 and 77 mEq/L and can contain other additional anions and cations to replaces the daily losses (Table 9.4). Table 9.4 gives an overview of the different hypotonic solutions. The main indication is to deliver free water in case of cellular dehydration. Recent evidence from the MIHMOSA and TOPMAST trials show that hypotonic balanced maintenance solutions are preferred over isotonic ones since they will lead to a less positive fluid and sodium balance.
Hypertonic Crystalloids
Hypertonic Saline
Hypertonic saline refers to any saline having concentration greater than 0.9% saline. These hypertonic crystalloids are available in varying concentrations ranging from 2% to 23% saline, but the commonly prescribed is 3% saline. Hypertonic saline 3% is indicated in critically ill for small-volume resuscitation, in patients with severe hyponatremia presenting with seizures or altered sensorium, and in patients of serve traumatic brain injury (having features of raised intracranial hypertension). Hypertonic saline exerts an osmotic effect by drawing fluid out of edematous tissues, as it has a higher concentration of sodium compared to interstitium.
Hypertonic saline acts on various body systems in different ways: Firstly, it affects hemodynamics by raising mean arterial pressure, raising cardiac output and stroke volume; it also increases left ventricular end-diastolic volume and reduces pulmonary vascular resistance. Secondly, it increases the total plasma volume and plasma vasopressin concentrations due to increased plasma osmolality. Thirdly, neurologic effects are related to increases in plasma osmolarity, and higher sodium concentration causes blood to be hypertonic compared to cerebral tissue (which has low sodium concentration). This difference leads to an osmotic gradient promoting the flow of excess water to move out of cerebral tissue. Trials showed an ICP improvement for approximately 72 h when sodium levels were increased by 10–15 mEq/l with hypertonic saline therapy [26]. Hypertonic saline increases capillary vessel inner diameter and plasma volume counteracting vasospasm and hypoperfusion by increased cerebral blood flow. These fluids have immune modulation and neurochemical properties too.
Hypertonic saline can be administered as bolus or continuous infusion in traumatic brain injury. The target serum osmolarity is less than 320 mOsmol/L. When treating patients of increased intracranial pressure with continuous 3% saline infusion, the optimal therapy is monitored by sodium levels and targeted between 145 and 155 mEq/l [27]. Hypertonic saline can be used as bolus in emergency situations, in concentrations ranging from 1.7% to 30% saline, and most often as bolus doses of 250 ml. The serum sodium level should be measured within 6 h of administration of bolus doses given. Readministration of hypertonic saline should not occur until the serum sodium concentration is <155 mEq/l. In head injury, when used in infusion, the rate of infusion has varied from 30 to 150 ml/h in light of the sodium levels.
For correction of severe hyponatremia, hypertonic saline is used in form of infusion guided by the sodium deficit calculated as total body water × wt (kg) × (desired sodium − actual sodium). The rate of sodium correction should be 6–12 mEq/L (0.5 mEq/h) in the first 24 h and 18 mEq per L or less in 48 h. A bolus of 100–150 mL of hypertonic 3% saline can be given to correct severe symptomatic hyponatremia until sodium levels reach 120 mEq/L. Limited evidence-supported resuscitation with 3% saline can reduce the total volume infused, less postoperative complications, and short ICU stay [28]. However, a recent a monocentric RCT failed to found any significant reduction in volume infused with the resuscitation using 7.3% saline vs 0.9% sodium chloride in postoperative cardiac surgery patients. Besides, a transient but considerable electrolytes and acid–base disturbance was noted in the hypertonic saline group [29].
The most serious potential complication of hypertonic saline administration is central pontine myelinolysis, characterized by a rapid and irreversible demyelination of the pons. Acute renal insufficiency has been seen in patients with traumatic brain injury receiving hypertonic saline, and this can be minimized by maintaining euvolemic state in such patients.
In comparison to mannitol, hypertonic saline causes less “rebound” ICP and lower nephrotoxicity, has no obligatory osmotic diuresis, and can be easily monitored by serial sodium levels.
Sodium Bicarbonate Solution
Bicarbonate is the leading source of CO2 transport in the plasma. However, the regulation of bicarbonate is mainly through the kidneys via secretion and absorption. Sodium bicarbonate (NaHCO3) is the most frequently used buffer to prevent or treat the metabolic acidosis and treat severe hyperkalemia. NaHCO3 is available in various forms: oral tablets, IV injections, and IV infusions. Injectable sodium bicarbonate is mainly available in two concentrations: 7.5% (44.6 mEq NaHCO3) and 8.5% (50 mEq NaHCO3). Injectable NaHCO3 has high osmolality and can cause thrombophlebitis with prolonged peripheral venous administration. However, bicarbonate administration can stimulate release of proinflammatory cytokines, superoxide radical production, apoptosis, and paradoxical intracellular acidosis due to production of CO2.
Indications of NaHCO3
-
Hyperkalemia (>6 mEq/l) and arrhythmias, especially in the setting of resuscitation
-
Alkalization in salicylate intoxication
-
Alkalization is essential (urinary pH 7.5–8.0, arterial <7.6).
-
Higher renal excretion, less fat soluble and less penetration by blood–brain barrier (BBB)
-
No standard dose: 1–2 mEq/kg bolus + maintenance infusion
-
-
Alkalinization in rhabdomyolysis [30]
Alkalinization of urine (pH> 6.5) prevents myoglobin casts formation and helps in AKI prevention. No RCTs compared NaHCO3 infusion to “classic” IV hydration. The criteria of rhabdomyolysis for NaHCO3 administration is a creatinine kinase (CK) increase to 5 times the upper limit of normal value. Start NaHCO3 from CK >10,000.
-
Sodium-channel blocker toxicity (e.g., tricyclic antidepressants)
-
Mainly based on animal experiments and case reports
Bolus 8.4% NaHCO3 1–2 mEq/kg on electrocardiogram abnormalities, malignant ventricular arrhythmias, or hemodynamic instability followed by maintenance infusion
-
-
During CVVH or intermittent hemodialysis for metabolic correction to a base excess of 0 to −5 (with or without substitution fluid or as a separate SPP)
-
Prevention of contrast-induced nephropathy (CIN) [31]
-
The use of NaHCO3 may reduce the risk of CIN (serum creatinine may increase by 0.5 mEq/l or increase by 25%), but no effect on the need of new dialysis or mortality.
-
Moderate heterogeneity, varying study patients, setting, and type of contrast agent.
-
More effect with emergency scans. More effect in studies published before 2008. The bolus is better than continuous infusion and in combination with N-acetylcysteine (NAC).
-
A meta-analysis of 125 studies favored NaHCO3 infusion over 0.9% saline along with N-acetylcysteine, vitamin C, statins, and adenosine antagonists for prevention of CIN after coronary angiography [32].
-
-
Another meta-analysis including 21,450 patients from 107 studies reported saline and N-acetylcysteine are the most effective treatment options that can reduce short-term mortality. However, none of the drugs could reduce the requirement of RRT or adverse cardiovascular events [33]. However, a recent RCT found no benefit of NaHCO3 infusion over saline in reduction of CIN, mortality, or need of RRT, after coronary angiography [34]. Metabolic acidosis with normal anion gap, correcting base excess to 0-5 (e.g., pronounced gastrointestinal loss, renal tubular acidosis) [35].
-
Dose = 0.3 × weight × −BE
-
-
NaHCO3 in the management of metabolic acidosis with high anion gap has been a matter of debate since long (BE of <−10 [aim is to achieve homeostasis of the internal environment as soon as possible]) [36, 37].
-
The multicenter open-label (BICAR-ICU) RCT evaluated the use of NaHCO3 infusion (vs no infusion) in critically ill patients with metabolic acidosis (pH ≤7.20) to target a pH >7.3. Sepsis and AKI were present in 61% and 47% of patients, respectively. Patients were sick with 83% on invasive mechanical ventilation and 80% on vasopressors. There was no benefit with NaHCO3 in the primary outcome, composite of mortality from any cause at day 28, and one or more organ failure at day 7. However, in subgroup of patients with AKI, the NaHCO3 infusion produced significant difference in composite outcome and individual components of mortality and organ failure [38].
Recent Surviving Sepsis Campaign guidelines suggested against using sodium bicarbonate therapy for the purpose of improving hemodynamics or reducing vasopressor requirements in patients with septic shock and hypoperfusion-induced lactic acidemia [39]. (Low quality of evidence). The following are side effects of NaHCO3:
-
Sodium overload (166.6 mmol/L)
-
fluid overload
-
Hypokalemia
-
Hyperosmolality: cellular dehydration
-
Hypocalcemia
-
Metabolic alkalosis: vasoconstriction
-
Extravasation
-
Very careful use in elderly patients
-
Possible pCO2 increase due to CO2 accumulation
Case Vignette
-
Q1. What will be the most appropriate fluid for initial resuscitation of this patient?
-
Recent studies have shown that balanced crystalloids are the best first choice in this setting. In case of profound shock and liver failure, exogenous lactate (from Ringer’s lactate) may accumulate. Hence, serum lactate values may lose their ability to discriminate between ongoing lactate production (DO2/VO2 mismatch) and diminished lactate clearance. Therefore, balanced crystalloids not containing lactate may be preferred (e.g., Plasma-Lyte).
-
Q2. Which fluid to choose for maintenance intravenous therapy now?
-
The best maintenance fluid is the one that has not been administered. One should only start maintenance solutions if the patient is not able to cover his/her daily fluid needs (25 ml/kg/day) orally or enterally. Recent studies showed that over 30% of the total fluid amount administered comes from fluids given to deliver antibiotics, pain killers, or other drugs. This is called fluid creep and should be reduced to a minimum. Fluid creep is defined as the sum of the volumes of electrolytes, the small volumes to keep venous lines open (saline or glucose 5%) and the total volume used as a vehicle for medication.
Conclusion
Crystalloids are the most common used fluids for critically ill patients. Despite an ongoing debate, balanced crystalloids are the preferred resuscitation fluid in most patients, except those with traumatic brain injury. Crystalloids should be prescribed like any other ICU medication, and selecting the right fluid, indication, dose, and duration is crucial for optimal outcomes.
Take Home Messages
-
Crystalloids like other medications should be used in the right patient, right indication, right dose, and for right duration.
-
Balanced crystalloids are resuscitation fluids of choice in patients with sepsis and septic shock, burns, or diabetic ketoacidosis.
-
0.9% sodium chloride is not normal nor physiological and its administration may cause harm in critically ill patients.
-
Hypertonic saline is mainly used for the treatment of raised intracranial pressure and symptomatic hyponatremia.
-
Maintenance solutions should be only considered if the patient is not able to cover his/her daily fluid needs orally or enterally.
-
Evidence does not support the use sodium bicarbonate therapy in patients with septic shock and hypoperfusion-induced lactic acidemia to support hemodynamics.
References
Latta TA. Malignant cholera. Documents communicated by the Central Board of Health, London, relative to the treatment of cholera by the copious injection of aqueous and saline fluids into the veins. Lancet. 1832;18:274–80.
Ringer S. Regarding the action of the hydrate of soda, hydrate of ammonia, and the hydrate of potash on the ventricle of the frog’s heart. J Physiol. 1880;3:195–202.
Hartmann AF, Senn MJ. Studies in the metabolism of sodium R-lactate. I. Response of normal human subjects to the intravenous injection of sodium R-lactate. J Clin Invest. 1932;11:327–35.
Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312–26.
Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384–94.
NICE. Intravenous fluid therapy in adults in hospital. London: National Institute for Health and Care Excellence; 2013. https://www.nice.org.uk/guidance/cg174.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77.
Rehm M, Finsterer U. Treating intraoperative hyperchloremic acidosis with sodium bicarbonate or tris-hydroxymethyl aminomethane: a randomized prospective study. Anesth Analg. 2003;96:1201–8.
Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasmalyte-148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24.
O’Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, Bennett-Guerrero E. A randomized, double-blind comparison of lactated Ringer’s solution and 0.9% NaCl during renal transplantation. Anesth Analg. 2005;100:1518–24.
Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury? Con. Kidney Int. 2014;86:1096–105.
Cull DL, Lally KP, Murphy KD. Compatibility of packed erythrocytes and Ringer’s lactate solution. Surg Gynecol Obstet. 1991;173:9–12.
Ergin B, Kapucu A, Guerci P, Ince C. The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br J Anaesth. 2016;117:521–8.
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal versus chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. J Am Med Assoc. 2012;308:1566–72.
Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–64.
Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91.
Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit. The SPLIT randomized clinical trial. JAMA. 2015;314:1701–10.
Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819–28.
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–39.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021;326(9):1–12.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021;326(9):830–8.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Association between type of fluid received prior to enrollment, type of admission, and effect of balanced crystalloid in critically ill adults: a secondary exploratory analysis of the BaSICS clinical trial. Am J Respir Crit Care Med. 2022;205:1419–28.
Jackson KE, Wang L, Casey JD, Bernard GR, Self WH, Rice TW, et al. Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest. 2021;159(2):585–95.
Finfer S, Micallef S, Hammond N, Navarra L, Bellomo R, Billot L, Delaney A, et al. Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med. 2022;386(9):815–26.
Hammond NE, Zampieri FG, Di Tanna GL, Garside T, Adigbli D, Cavalcanti AB, et al. Balanced crystalloids versus saline in critically ill adults – a systematic review with meta-analysis. NEJM Evid. 2022;1(2):10. https://doi.org/10.1056/EVIDoa2100010.
Khanna S, Davis D, Peterson B, Fisher B, Tung H, O’Quigley J, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–51.
Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, et al. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: Effect on intracranial pressure and lateral displacement of the brain. Crit Care Med. 1998;26(3):440–6.
Pfortmueller CA, Schefold JC. Hypertonic saline in critical illness - a systematic review. J Crit Care. 2017;42:168–77.
Pfortmueller CA, Kindler M, Schenk N, Messmer AS, Hess B, Jakob L, et al. Hypertonic saline for fluid resuscitation in ICU patients post-cardiac surgery (HERACLES): a double-blind randomized controlled clinical trial. Intensive Care Med. 2020;46(9):1683–95.
Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20(1):135. https://doi.org/10.1186/s13054-016-1314-5.
Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. https://doi.org/10.1136/bmj.h4395.
Ali-Hasan-Al-Saegh S, Mirhosseini SJ, Ghodratipour Z, Sarrafan-Chaharsoughi Z, Rahimizadeh E, Karimi-Bondarabadi AA, et al. Strategies preventing contrast-induced nephropathy after coronary angiography: a comprehensive meta-analysis and systematic review of 125 randomized controlled trials. Angiology. 2017;68(5):389–413.
Ma WQ, Zhao Y, Wang Y, Han XQ, Zhu Y, Liu NF. Comparative efficacy of pharmacological interventions for contrast-induced nephropathy prevention after coronary angiography: a network meta-analysis from randomized trials. Int Urol Nephrol. 2018;50(6):1085–95.
Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378(7):603–14.
Adeva-Andany MM, Fernandez-Fernandez C, Mourino-Bayolo D, Castro-Quintela E, Dominguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. Sci World J. 2014;2014:627673. https://doi.org/10.1155/2014/627673, indexed in Pubmed: 25405229.
Velissaris D, Karamouzos V, Pierrakos C, Koniari I, Apostolopoulou C, Karanikolas M. Use of sodium bicarbonate in cardiac arrest: current guidelines and literature review. J Clin Med Res. 2016;8(4):277–83.
Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(1):94–102.
Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392(10141):31–40.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Singh, A., Chawla, A. (2024). The Place of Crystalloids. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)