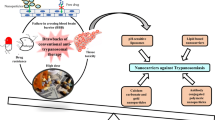
Abstract
Trypanosomiasis is a neglected tropical disease that is mainly prevalent in low- and middle-income countries including regions of Africa, Asia, and America. Except for fexinidazole which was discovered in late 2018, no new drug has been discovered in the last 50 years for the treatment of trypanosomiasis. Furthermore, emergence of drug resistance against FDA-approved antitrypanosomal drugs has also significantly affected the therapy. Nanotechnological interventions of the existing drugs have shown significant improvement in the therapeutic potential of FDA-approved drugs in preclinical research. The chapter focuses on the nanomedicines-based treatment of trypanosomiasis. A brief discussion on vaccine delivery against trypanosome has also been included.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption distribution metabolism excretion
- ApoE:
-
Apolipoprotein E
- apoL-1:
-
Apolipoprotein
- CCL5:
-
CC-chemokine ligand 5
- CD8:
-
Cluster of differentiation 8
- CNS:
-
Central Nervous System
- CPP:
-
Cell-penetrating peptides
- DNA:
-
Deoxyribonucleic acid
- FDA:
-
Food and Drug Administration
- GPI:
-
Glycosylphosphatidylinositol
- IC50:
-
Inhibitory Concentration
- IFN:
-
Interferons
- IgG:
-
Immunoglobulin G
- IL:
-
Interleukin
- IM:
-
Intramuscular
- IV:
-
Intravenous
- LDL:
-
Low-Density Lipoprotein
- NECT:
-
Nifurtimox–eflornithine combination therapy
- PEG:
-
Polyethylene Glycol
- PLGA:
-
Poly(lactic-co-glycolic acid)
- RBC:
-
Red Blood Cells
- ROS:
-
Reactive oxygen species
- SC:
-
Subcutaneous
- SLN:
-
Solid Lipid Nanoparticles
- SRA:
-
Serum Resistance Protein
- Tc24:
-
Trypomastigote excretory-secretory protein
- TGO:
-
Glutamic-oxaloacetic transaminase
- TGP:
-
Glutamic Pyruvic Transaminase
- TNF:
-
Tumor necrosis factor
- Tr-apoL-I:
-
Truncated apoL-I
- TSA-1:
-
Trypomastigote surface trans-sialidase
- Vd:
-
Volume of distribution
- VSGs:
-
Variant Surface Glycoprotein
- WHO:
-
World Health Organization
References
Prayag K, Surve DH, Paul AT, Kumar S, Jindal AB. Nanotechnological interventions for treatment of trypanosomiasis in humans and animals. Drug Deliv Transl Res. 2020;10:945–61.
WHO Trypansomiasis. WHO-Trypansomiasis, international travel & health issue [Internet]. [cited 2019 Jul 10]. Available from: https://www.who.int/ith/diseases/trypanosomiasis/en/.
Kirchoff VL. Chagas disease (American trypanosomiasis). Medscape. 2018:1–31. Available from: https://emedicine.medscape.com/article/214581-overview?form=fpf#a4
WHO fact sheet on Chagas disease (American trypanosomiasis). Available from: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
de Rezende JM, Rassi A. American trypanosomiasis (Chagas disease). Infect Dis Clin. 2012;26:275–91.
Pan America Health Organization W. Guidelines for the diagnosis and treatment of Chagas disease. Washington, D.C.: PAHO; 2019.
WHO-American trypansomiasis. Available from: https://www.who.int/chagas/epidemiology/en/.
Simarro PP, Jannin J, Cattand P. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 2008;5:174–80.
WHO. Trypanosomiasis, human African (sleeping sickness) [Internet]. [cited 2022 Jul 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness).
Babokhov P, Sanyaolu AO, Oyibo WA, Fagbenro-beyioku AF, Iriemenam NC. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog Glob Health. 2013;107:242–52.
WHO Report on trypansomiasis. Control and surveillance of human African trypanosomiasis 2001. World Health Organization; 2013.
Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–96.
Chagas Disease [Internet]. Centers Dis. Control Prev [cited 2020 Jan 18]. Available from: https://www.cdc.gov/parasites/chagas/index.html.
Centers for Disease Control and Prevention [Internet]. African trypanos [cited 2020 Jan 18]. Available from: https://www.cdc.gov/parasites/sleepingsickness/index.html.
Landfear SM. Nutrient transport and pathogenesis in selected parasitic protozoa. Eukaryot Cell. 2011;10:483–93.
Nenarokova A, Michels PAM. A paradigm shift: the mitoproteomes of procyclic and bloodstream Trypanosoma brucei are comparably complex. PLoS Pathog. 2017;13:1–9.
Tiengwe C, Bush PJ, Bangs JD. Controlling transferrin receptor trafficking with GPI-valence in bloodstream stage African trypanosomes. PLoS Pathog [Internet]. Public Library of Science. 2017 [cited 2022 Jul 6];13:e1006366. Available from: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006366.
Mugnier MR, Stebbins CE, Papavasiliou FN. Masters of disguise: antigenic variation and the VSG coat in Trypanosoma brucei. PLoS Pathog. 2016;12:1–6.
Glover L, Hutchinson S, Alsford S, Mcculloch R, Field MC, Horn D. Antigenic variation in African trypanosomes: the importance of chromosomal and nuclear context in VSG expression control. Cell Microbiol. 2013;15:1984–93.
De Souza W. Basic cell biology of Trypanosoma cruzi. Curr Pharm Des. 2002;8:269–85.
De Koning HP. Transporters in African trypanosomes: role in drug action and resistance. Int J Parasitol. 2001;31:512–22.
Quemé-Peña M, Ricci M, Juhász T, Horváti K, Bösze S, Biri-Kovács B, et al. Old polyanionic drug suramin suppresses detrimental cytotoxicity of the host defense peptide LL-37. ACS Pharmacol Transl Sci. 2021;4:155–67.
Yun O, Priotto G, Tong J, Flevaud L. NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis. 2010;4:1–5.
Samo M, Jannin JG. Monitoring the use of nifurtimox-eflornithine combination therapy (NECT) in the treatment of second stage gambiense human African trypanosomiasis. Res Rep Trop Med. 2012;3:93–101.
Salomon CJ. First century of Chagas’ disease: an overview on novel approaches to nifurtimox and benznidazole delivery systems. J Pharm Sci. 2012;101:888–94.
Rassi A, Marin-Neto JA. Chagas disease. Lancet. Elsevier. 2010;375:1388–402.
Deeks ED. Fexinidazole: first global approval. Drugs Drugs. 2019;79:215–20.
Baker N, De Koning HP, Ma P, Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–8.
Application to include nifurtimox-eflornithin combination as treatment for stage 2 trypanosoma brucei gambiense human african trypanosomiasis (sleeping sickness) on the 4th who model list of essential medicines for children submitted by Drugs for Neglected Diseases initiative (DNDi), Geneva, Switzerland on 29 November, 2012.
Pearce L. Studies on the treatment of human trypanosomiasis with tryparsamide (the sodium salt of N-phenylglycineamide-p-arsonic acid). J Exp Med. The Rockefeller University Press. 1921;34:1.
Road B. The pharmacology of isometamidium. J Vet Pharmacol Therp. 1988;11:233–45.
Yashica Pharmaceuticals Pvt. Ltd. Product information-Quinapyramine chloride/sulphate BP [Internet]. [cited 2020 Nov 29]. Available from: https://www.pharmarawmaterials.com/quinapyramine-chloride-sulphate.html.
Kroubi M, Karembe H, Betbeder D. Drug delivery systems in the treatment of African trypanosomiasis infections. Expert Opin Drug Deliv. 2011;8:735–47.
Rodgers J, Jones A, Gibaud S, Bradley B, McCabe C, Barrett MP, et al. Melarsoprol cyclodextrin inclusion complexes as promising oral candidates for the treatment of human African trypanosomiasis. PLoS Negl Trop Dis. 2011;5:e1308.
Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. NIH Public Access. 2013;172:782.
Olbrich C, Gessner A, Schro W, Kayser O, Mu RH. Lipid – drug conjugate nanoparticles of the hydrophilic drug diminazene — cytotoxicity testing and mouse serum adsorption. J Control Release. 2004;96:425–35.
Esteva I, Scalise L, Arru EC, Rial MS, Salomon J, Fichera LE. Elucidating the impact of low doses of nano- formulated benznidazole in acute experimental Chagas disease. PLoS Negl Trop Dis. 2017;11:1–16.
Morilla MJ, Montanari JA, Prieto MJ, Lopez MO, Petray PB, Romero EL. Intravenous liposomal benznidazole as trypanocidal agent: increasing drug delivery to liver is not enough. Int J Pharm. 2004;278:311–8.
Scalise ML, Arrúa EC, Rial MS, Esteva MI, Salomon CJ, Fichera LE. Promising efficacy of benznidazole nanoparticles in acute Trypanosoma cruzi murine model: in-vitro and in-vivo studies. Am Soc Trop Med Hyg. 2016;95:388–93.
Gonzalez-martin G, Mkrino I, Rodriguez-cabezas. Characterization and trypanocidal activity of nifurtimox-containing and empty nanoparticles of polyethylcyanoacrylates. J Pharm Pharmacol. 1998;50:29–35.
Abriata JP, Eloy JO, Riul TB, Campos PM, Baruffi MD, Marchetti JM. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mater Sci Eng C. Elsevier. 2017;77:1196–203.
Gonza G. Allopurinol encapsulated in polycyanoacrylate nanoparticles as potential lysosomatropic carrier: preparation and trypanocidal activity. Eur J Pharm Biopharm. 2000;49:137–42.
Manuja A, Kumar B, Chopra M, Bajaj A, Kumar R. Cytotoxicity and genotoxicity of a trypanocidal drug quinapyramine sulfate loaded-sodium alginate nanoparticles in mammalian cells. Int J Biol Macromol. Elsevier B.V. 2016;88:146–155.
Manuja A, Kumar S, Dilbaghi N, Bhanjana G, Chopra M, Kaur H, Kumar R, Manuja B, Singh SYS. Quinapyramine sulfate-loaded sodium alginate nanoparticles show enhanced trypanocidal activity. Nanomedicine. 2014;9:1625–34.
Surve DH, Jindal AB. Development of cationic Isometamidium chloride loaded long-acting lipid nanoformulation: optimization, cellular uptake, pharmacokinetics, biodistribution, and immunohistochemical evaluation. Eur J Pharm Sci. Elsevier. 2021;167:106024.
Kroubi M, Daulouede S, Karembe H. Development of a nanoparticulate formulation of diminazene to treat African trypanosomiasis. Nanotechnology. 2010;505102:1–8.
Manuja A, Kumar B, Chopra M, Bajaj A, Kumar R, Dilbaghi N, Kumar S, Singh S, Riyesh T YS. Cytotoxicity and genotoxicity of a trypanocidal drug quinapyramine sulfate loaded-sodium alginate nanoparticles in mammalian cells. Int J Biol Macromol. Elsevier B.V. 2016;88:146–155.
Gilbert IH. Target-based drug discovery for human African trypanosomiasis: selection of molecular target and chemical matter. Parasitology. Cambridge University Press. 2014;141:28.
Navya PN, Daima HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. Springer. 2016;3:1.
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, et al. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett. Springer. 2020;15:1–14.
Bhatt R, Goyal A, Kachhwaha S, Kothari SL. Capping of nanoparticles: an alternative approach for reducing nanoparticle toxicity in plants; submitted in Material Science (Cornell University), 22 Nov. 2022
Tewabe A, Abate A, Tamrie M, Seyfu A, Siraj EA. Targeted drug delivery — from magic bullet to nanomedicine: principles, challenges, and future perspectives. J Multidiscip Healthc Dove Press. 2021;14:1711.
Delespaux V, De Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat. 2007;10:30–50.
Garcia-Salcedo JA, Unciti-Broceta JD, Valverde-Pozo J, Soriano M. New approaches to overcome transport related drug resistance in trypanosomatid parasites. Front Pharmacol. Frontiers Media SA. 2016;7:351.
Pinger J, Chowdhury S, Papavasiliou FN. Variant surface glycoprotein density defines an trypanosomes undergoing antigenic variation. Nat Commun. 2017;8:1–9.
Unciti-broceta JD, Arias JL, Maceira J, Soriano M. Specific cell targeting therapy bypasses drug resistance mechanisms in African trypanosomiasis. PLoS Pathog. 2015;11:1–20.
Unciti JD, Del T. Novel therapy based on camelid nanobodies. Ther Deliv. 4:1321–36.
Conrath K, Vanhollebeke B, Pays E, Baral TN, Magez S, Muyldermans S, et al. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat Med. 2006;12:580–4.
Dewar S, Sienkiewicz N, Ong HB, Wall RJ, Horn D, Fairlamb AH. The role of folate transport in antifolate drug action in Trypanosoma brucei. J Biol Chem. 2016;291:24768–78.
Kariuki CK, Stijlemans B, Magez S. The trypanosomal transferrin receptor of Trypanosoma Brucei—a review. Trop Med Infect Dis. Multidisciplinary Digital Publishing Institute (MDPI). 2019;4:126.
Trevor CE, Gonzalez-Munoz AL, Macleod OJS, Woodcock PG, Rust S, Vaughan TJ, et al. Structure of the trypanosome transferrin receptor reveals mechanisms of ligand recognition and immune evasion. Nat Microbiol. 2019;4:2074–81.
Arrighi RBG, Ebikeme C, Jiang Y, Ranford-Cartwright L, Barrett MP, Langel Ü, et al. Cell-penetrating peptide TP10 shows broad-spectrum activity against both Plasmodium falciparum and Trypanosoma brucei brucei. Antimicrob Agents Chemother. 2008;52:3414–7.
Yoo J-W, Chambers E, Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des. 2010;16:2298–307.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. NIH Public Access. 2015;33:941.
Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed. China Humanity Technology Publishing House. 2014;4:S1.
Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. SpringerOpen. 2018;13:1–12.
Kumari A, Singla R, Guliani A, Yadav SK. Nanoencapsulation for drug delivery. EXCLI J. Leibniz Research Centre for Working Environment and Human Factors. 2014;13:265.
Omarch G, Kippie Y, Mentor S, Ebrahim N, Fisher D, Murilla G, et al. Comparative in vitro transportation of pentamidine across the blood-brain barrier using polycaprolactone nanoparticles and phosphatidylcholine liposomes. Artif Cells Nanomed Biotechnol. 2019;47:1428–36.
Samuelsson E, Shen H, Blanco E, Ferrari M, Wolfram J. Contribution of Kupffer cells to liposome accumulation in the liver. Colloids Surf B Biointerfaces. NIH Public Access. 2017;158:356.
Morilla MJ, Montanari J, Frank F, Malchiodi E, Corral R, Patricia Petray ELR. Etanidazole in pH-sensitive liposomes: design, characterization and in vitro/in vivo anti-Trypanosoma cruzi activity. J Control Release. 2005;103:599–607.
Antimisiaris SG, Ioannou PV, Loiseau PM. In-vitro antileishmanial and trypanocidal activities of arsonoliposomes and preliminary in-vivo distribution in BALB/c mice. J Pharm Pharmacol. 2003;55:647–52.
Melarsoprol toxicity in the treatment of human African trypanosomiasis. Ten cases treated with dimercaprol [Internet]. [cited 2022 Jul 22]. Available from: https://pubmed.ncbi.nlm.nih.gov/3252975/.
MELARSOPROL injectable | MSF Medical Guidelines [Internet]. [cited 2022 Jul 22]. Available from: https://medicalguidelines.msf.org/en/viewport/EssDr/english/melarsoprol-injectable-16682915.html.
Fairlamb AH, Horn D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. Elsevier Ltd. 2018;34:481–492.
Zirar Ben S, Astier A, Muchow M, Gibaud S. Comparison of nanosuspensions and hydroxypropyl- b -cyclodextrin complex of melarsoprol: pharmacokinetics and tissue distribution in mice. Eur J Pharm Biopharm. 2008;70:649–56.
Simarro PP, Franco J, Diarra A, Postigo JAR, Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139:842–6.
Arias JL, Unciti-broceta JD, Maceira J, Castillo T, Hernández-quero J, Magez S, et al. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J Control Release. 2015;197:190–8.
Tachibana H, Yoshihara E, Kaneda Y, Nakae T. In vitro lysis of the bloodstream forms of Trypanosoma brucei gambiense by stearylamine-bearing liposomes. Antimicrob Agents Chemother. 1988;32:966–70.
Singh S, Chopra M, Dilbaghi N, Manuja B, Kumar S, Kumar R, Rathore N, Yadav SMA. Synthesis and evaluation of isometamidium-alginate nanoparticles on equine mononuclear and red blood cells. Int J Biol Macromol. Elsevier BV. 2016;92:788–794.
Prayag KS, Paul AT, Ghorui SK, Jindal AB. Preparation and evaluation of quinapyramine sulphate-docusate sodium ionic complex loaded lipidic nanoparticles and its scale up using geometric similarity principle. J Pharm Sci. Elsevier Ltd. 2021;110:2241–2249.
Tessarolo LD, Róseo R, Pessoa P, de Menezes B, Mello CP, Lima DB, et al. Nanoencapsulation of benznidazole in calcium carbonate increases its selectivity to Trypanosoma cruzi. Parasitology. 2018;145:1191–8.
Carneiro ZA, Maia PIS, Sesti-costa R, Lopes CD, Pereira TA, Silva S, et al. In vitro and in vivo trypanocidal activity of H 2 bdtc-loaded solid lipid nanoparticles. PLoS Negl Trop Dis. 2014;8:e2847.
Molina J, Urbina J, Gref R, Brener Z, Maciel J, Júnior R. Cure of experimental Chagas’ disease by the bis-triazole D0870 incorporated into ‘stealth’ polyethyleneglycol – polylactide nanospheres. J Antimicrob Chemother. 2001;47:101–4.
Andrade-Neto VF, Brandão MGL, Stehmann JR, Oliveira LA, Krettli AU. Antimalarial activity of Cinchona-like plants used to treat fever and malaria in Brazil. J Ethnopharmacol [Internet]. 2003 [cited 2022 Jun 30];87:253–6. Available from: https://pubmed.ncbi.nlm.nih.gov/12860318/.
Wu D, Kong Y, Han C, Chen J, Hu L, Jiang H, et al. d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int J Antimicrob Agents. Elsevier. 2008;32:421–426.
Del Prado-Audelo ML, Cortés H, Caballero-Florán IH, González-Torres M, Escutia-Guadarrama L, Bernal-Chávez SA, et al. Therapeutic applications of terpenes on inflammatory diseases. Front Pharmacol. Frontiers Media S.A. 2021;12:2114.
Cimmino A, Roscetto E, Masi M, Tuzi A, Radjai I, Gahdab C, et al. Sesquiterpene lactones from Cotula cinerea with antibiotic activity against clinical isolates of Enterococcus faecalis. Antibiotics. 2021;10:819 [Internet]. Multidisciplinary Digital Publishing Institute. Available from: https://www.mdpi.com/2079-6382/10/7/819/htm.
Hoet S, Opperdoes F, Brun R, Quetin-Leclercq J, Opperdoes F. Natural products active against African trypanosomes: a step towards new drugs. Nat Prod Rep. 2004;21:353–64.
Uchiyama N. Antichagasic activities of natural products against Trypanosoma cruzi. J Health Sci. 2009;55:31–9.
Cretton S, Breant L, Pourrez L, Ambuehl C, Marcourt L, Ebrahimi SN, et al. Antitrypanosomal quinoline alkaloids from the roots of waltheria indica. J Nat Prod. American Chemical Society. 2014;77:2304–2311.
Ngantchou I, Nyasse B, Denier C, Blonski C, Hannaert V, Schneider B. Antitrypanosomal alkaloids from Polyalthia suaveolens (Annonaceae): their effects on three selected glycolytic enzymes of Trypanosoma brucei. Bioorganic Med Chem Lett. 2010;20:3495–8.
Sanchez LM, Knudsen GM, Helbig C, De Muylder G, Mascuch SM, MacKey ZB, et al. Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J Nat Prod. 2013;76:630–41.
Takeara R, Albuquerque S, Lopes NP, Callegari Lopes JL. Trypanocidal activity of Lychnophora staavioides Mart. (Vernonieae, Asteraceae). Phytomedicine. Urban und Fischer Verlag Jena. 2003;10:490–493.
Martínez-Luis S, Félix Gómez J, Spadafora C, Guzmán HM, Gutiérrez M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules [Internet]. 2012;17:11146–55. Available from: www.mdpi.com/journal/moleculesArticle.
Inahashi Y, Iwatsuki M, Ishiyama A, Namatame M, Nishihara-Tsukashima A, Matsumoto A, et al. Spoxazomicins A-C, novel antitrypanosomal alkaloids produced by an endophytic actinomycete, Streptosporangium oxazolinicum K07-0460T. J Antibiot (Tokyo). 2011;64:303–7.
Kwofie KD, Tung NH, Suzuki-Ohashi M, Amoa-Bosompem M, Adegle R, Sakyiamah MM, et al. Antitrypanosomal activities and mechanisms of action of novel tetracyclic iridoids from Morinda lucida Benth. Antimicrob Agents Chemother. American Society for Microbiology. 2016;60:3283–3290.
Baldissera MD, Grando TH, De Souza CF, Cossetin LF, Silva APT, Giongo JL, et al. A nanotechnology based new approach for Trypanosoma evansi chemotherapy: in vitro and vivo trypanocidal effect of (-)-α-bisabolol. Exp Parasitol. 2016;170:156–60.
Rani R, Kumar S, Dilbaghi N, Kumar R. Nanotechnology enabled the enhancement of antitrypanosomal activity of piperine against Trypanosoma evansi. Exp Parasitol. Academic Press Inc. 2020;219:108018.
Ibezim A, Debnath B, Ntie-Kang F, Mbah J, et al. Binding of anti-Trypanosoma natural products from African flora against selected drug targets: a docking study. Med Chem Res. 2017;26:562–79.
Ritter CS, Baldissera MD, Grando TH, Souza CF, Sagrillo MR, da Silva APT, et al. Achyrocline satureioides essential oil-loaded in nanocapsules reduces cytotoxic damage in liver of rats infected by Trypanosoma evansi. Microb Pathog. Academic Press. 2017;103:149–154.
Branquinho RT, Roy J, Farah C, Garcia GM, Aimond F, Le Guennec J, et al. Biodegradable polymeric nanocapsules prevent cardiotoxicity of anti- trypanosomal lychnopholide. Sci Reports-Nature. 2017;7:1–13.
Baldissera MD, Da Silva AS, Oliveira CB, Santos RCV, Vaucher RA, Raffin RP, et al. Trypanocidal action of tea tree oil (Melaleuca alternifolia) against Trypanosoma evansi in vitro and in vivo used mice as experimental model. Exp Parasitol. Academic Press Inc. 2014;141:21–7.
Tabel H, Wei G, Bull HJ. Immunosuppression: cause for failures of vaccines against African trypanosomiases. PLoS Negl Trop Dis. Public Library of Science. 2013;7:e2090.
Stijlemans B, Radwanska M, De Trez C, Magez S. African trypanosomes undermine humoral responses and vaccine development: link with inflammatory responses? Front Immunol. Frontiers Research Foundation. 2017;8:582.
Autheman D, Crosnier C, Clare S, Goulding DA, Brandt C, Harcourt K, et al. An invariant Trypanosoma vivax vaccine antigen induces protective immunity. Nat Publ Group. 2021;595:96–100.
Bivona AE, Alberti AS, Cerny N, Trinitario SN, Malchiodi EL. Chagas disease vaccine design: the search for an efficient Trypanosoma cruzi immune-mediated control. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165658.
Dumonteil E, Bottazzi ME, Zhan B, Heffernan MJ, Jones K, Valenzuela JG, et al. Accelerating the development of a therapeutic vaccine for human Chagas disease: rationale and prospects. Expert Rev Vaccines. 2014;11:1043–55.
Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra MJ. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in Mice. Infect Immun. 2004;72:46–53.
Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. Frontiers Media SA. 2018;9:2224.
Bhardwaj P, Bhatia E, Sharma S, Ahamad N, Banerjee R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. Elsevier. 2020;108:1.
Acknowledgments
Authors are thankful to the Department of Biotechnology, Government of India, for providing financial support for the project (BT/PR18008/NNT/28/1055/2016 dated 17.09.2018).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Prayag, K.S., Jindal, A.B. (2023). Nanomedicines for the Treatment of Trypanosomiasis. In: Patravale, V.B., Date, A.A., Jindal, A.B. (eds) Nanomedicines for the Prevention and Treatment of Infectious Diseases. AAPS Advances in the Pharmaceutical Sciences Series, vol 56. Springer, Cham. https://doi.org/10.1007/978-3-031-39020-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-39020-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-39019-7
Online ISBN: 978-3-031-39020-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)