Abstract
Endocrine-disrupting chemicals (EDCs) are prevalent throughout the environment and known to negatively impact fertility. As the prevalence of unexplained subfertility increases worldwide, it is important to understand the impact EDCs might have on reproduction and fertility treatments, such as in vitro fertilisation (IVF). This chapter examines the impact of EDCs on assisted reproduction treatments and pregnancy outcomes. The literature is frequently conflicting; however, the association between exposure to EDCs and poor reproductive outcomes is undeniable. Women of a reproductive age should therefore minimise exposure to these chemicals where possible, with healthcare professionals actively advocating their avoidance where possible. Further research is needed to determine the exact mechanisms of action of these substances, to identify which specific chemicals have the greatest effect and to provide recommendations for how to mitigate these effects.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- EDCs
- Endocrine-disrupting chemicals
- BPA
- Phthalates
- Infertility
- IVF
- ART
- Reproductive outcomes
- Pregnancy outcomes
8.1 Introduction
Infertility can be defined as ‘a disease characterized by the failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse’. [1] In the past 50 years, there has been a steady yet undeniable rise of this condition, [2, 3] with one in six couples hoping to conceive being diagnosed as infertile [4]. There are many well-recognised causes of infertility, including advanced age, sexually transmitted infections, endocrine disorders such as polycystic ovarian syndrome and male factor [5,6,7]. However, in more than 10% of couples, the cause of infertility is unknown, [8] and the prevalence of such unexplained subfertility is increasing [7, 9]. In this condition, women have apparently normal ovulatory cycles, normal hormonal levels and no obvious reproductive disease. Likewise, their male partners have apparently normal semen quality, yet conception does not occur.
One explanation for this increase in unexplained subfertility could be the increasing prevalence of endocrine-disrupting chemicals (EDCs). These are exogenous chemicals that have the ability to disturb the normal endocrine function of humans, [3] thus interfering with normal development, homeostasis and reproduction [10]. EDCs are a diverse group of substances used extensively within the manufacturing, industrial and agricultural industries [11]. The most important EDCs are bisphenol A (BPA), phthalates (both used in plastic manufacturing), triclosan (widely used in pharmaceuticals and personal care products) and parabens (used as food preservatives and in cosmetics). Human exposure to EDCs is almost ubiquitous as it takes many forms—mostly via ingestion, but also through inhalation or dermal uptake [12]. Once in the body, most are able to bioaccumulate within adipose tissue; their long half-life results in prolonged exposure to these substances [13]. Furthermore, EDCs are ubiquitous in nature, resulting in a combination of different chemicals accumulating; [14,15,16] these cumulative effects perhaps worse than those individually.
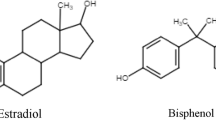
As the production of synthetic chemicals dramatically rises worldwide, [14, 17, 18] questions have arisen regarding their effects on human health, including reproductive health. It has been widely shown that environmental pollution negatively impacts the fertility of all mammalian species [1, 5]. However, in more recent years, substantial evidence has accumulated showing a negative association specifically between EDC exposure and both male and female fertility and fecundity [11, 15]. Animal studies have demonstrated that in females, ovulation, meiosis, the number of follicles present in the ovaries and embryonic implantation can all be impacted by such chemicals [1]. Whilst comparative effects are also seen in males—effects such as hypospadias and poor semen quality have been noted—the most severe consequences on fertility are seen in females; as the number of oocytes is fixed at birth, there is no possibility for replacement. Whilst the overall pathophysiological mechanisms of EDCs in relation to fertility are not fully understood, [16, 19] studies have shown that they are able to competitively bind with hormone receptors, exerting adverse biological functions. For example, BPA and triclosan have similar structure to 17𝛽-oestradiol [3] and are therefore able to interfere with oestrogen signalling pathways [11, 14].
Perhaps unsurprisingly, as the prevalence of infertility has risen worldwide, so too has demand for services providing assisted reproductive technology (ART), including in vitro fertilisation (IVF) [20,21,22]. Having established that EDCs negatively impact human fertility, it is prudent to understand their possible impact on fertility treatment. This chapter looks at the effects of specific EDCs on the outcomes of IVF treatment and other forms of ART, as well as their impact on the pregnancy outcomes.
8.2 Impact of EDCs on IVF Outcomes
8.2.1 Bisphenol A
Bisphenol A (BPA) is perhaps the most thoroughly examined EDC. Its extensive presence in everyday products including plastics, medical equipment, the epoxy lining of food and drink containers and dental sealants mean that the general population are widely exposed to its effects [23, 24]. Indeed, one study found up to 95% of patients tested had detectable levels of BPA in urine samples, [11] and it has also been detected in the follicular fluid of most women commencing IVF treatment [25]. Unlike other EDCs, BPA has a comparably short half-life and is not prone to bioaccumulation, with almost complete urinary excretion within 24 hours, [13, 15] though this does not mitigate its harmful effects; studies show correlations between increased levels of BPA in adult urine samples and increased incidence of obesity, cardiovascular disease and Type II diabetes mellitus [11].
In relation to fertility, it is thought BPA inhibits aromatase activity, thereby inhibiting oestrogen synthesis and disrupting ovarian folliculogenesis and implantation [24]. This therefore directly impacts not only female fertility but also the outcomes of any subsequent fertility treatment. Several studies have shown relationships between increased exposure to BPA in females and poor outcomes of IVF treatment: specifically, serum BPA levels have been negatively associated with oocyte retrieval, oocyte maturation, fertilisation rates and embryo quality [26,27,28].
One of the seminal studies in this field was the Environmental and Reproductive Health (EARTH) study. Researchers consistently found a correlation between levels of BPA in female urine samples and decreased peak serum oestradiol levels and the number of both total and mature oocytes retrieved [25, 29]. There was also an association between BPA level and the rate of normally fertilised oocytes, with a decrease of 24% and 27%, respectively, for the highest versus lowest quartiles of urinary BPA levels. Additionally, increased urinary BPA concentrations are associated with a reduced successful implantation rate, with these trends showing positive linear dose–response associations [30]. This finding has since been confirmed by multiple other studies [13, 26, 27, 31, 32]. Increased miscarriage rates, post-non successful treatment, have also been reported, [11, 13] alongside increased rates of premature birth [13].
Whilst ethical challenges make it difficult to carry out experimental studies in humans, evidence available from experimental animal studies suggests a similar picture. In female mice, sub-chronic low-dose BPA exposure has been linked to diminished ovarian reserve [33]. Other animal studies have suggested association between BPA and spindle abnormalities, as well as impairment of follicular growth and implantation [11, 32]. Alongside antagonisation of oestrogen pathways, BPA has been shown to alter animal uterine morphology and reduce uterine receptivity preimplantation, [18] as well as impairing steroidogenesis in rat ovarian theca-interstitial and granulosa cells in vitro [34]. The long-term effects of these are unknown, but potentially trans-generational [11].
However, despite the effects of BPA upon significant IVF outcomes including oocyte retrieval rate and implantation rate, the arguably more important outcomes of clinical pregnancy and live birth appear to be unaffected in some studies [15].
8.2.2 Phthalates
Phthalates are a class of synthetic chemicals used extensively as plasticisers and solvents and within food processing and personal care products [11, 24, 35,36,37]. As a group of substances, there is ever-increasing evidence regarding their effect on human fertility and fecundity, including IVF outcomes. Epidemiological evidence has suggested some women of reproductive age are at particular risk due to daily use of cosmetics and other personal care items [18, 36]. As with BPA, phthalates have a ubiquitous presence, evidenced by the presence of nine out of the 13 phthalate metabolites measured in the urine of more than 99% of people [38]. They are also detectable in amniotic fluid [11] and are able to cross via placental transfer to a developing foetus and via breastmilk to neonates [18]. This may have profound implications for the development of the endocrine system of the affected child, potentially impacting their later reproductive functions.
As with BPA outcomes, in women undergoing fertility treatment, phthalate levels are associated with increased risk of implantation failure [24, 39]. The isotope di-2-exthylhexyl phthalate (DEHP) is the most well-studied phthalate, due to its concerning anti-androgenic properties [24, 35, 37]. Increased urinary DEHP levels have been associated with: decreased antral follicle counts; [11] decreased total and mature oocyte yield during IVF; [11, 15, 23, 24] decreased oocyte quality [24] and reduced probability of clinical pregnancy and live birth following IVF [11, 15, 24]. These studies suggest phthalates have the worrying potential to lower the probability of clinical pregnancy and live birth following IVF treatment—unlike BPA, which only appears to affect earlier ART outcomes. Other phthalate isotypes have also been associated with poor IVF outcomes: monobenzyl phthalate (MBzP) and mono-n-butyl phthalate (MBP) concentrations are associated with decreased blastocyst quality, [37] and urinary monoethyl phthalate (MEP) levels were associated with clinical pregnancy loss and preterm birth in some studies (though other studies found no associations) [23].
Several animal studies provide further evidence of phthalates acting as toxicants to the female reproductive system; [15] they have been shown to disrupt ovarian function and negatively impact folliculogenesis and steroidogenesis [11, 24, 36]. In female rats, DEHP was associated with reduced serum oestradiol and progesterone levels [28, 35]. Likewise, in male rats, exposure to DEHP decreased androgen production and altered sexual differentiation [40]. However, unlike BPA, which has been found in higher concentrations in women requiring infertility treatment compared to those who do not, [26] there have been no associations found between increased urinary concentrations of phthalate metabolites and the need for fertility treatment [35].
A study by Du et al. (2019) found that the follicular fluid concentration of certain phthalate metabolites was associated with altered levels of intrafollicular reproductive hormones—including oestradiol and testosterone—in women undergoing IVF [36]. This is concerning, and the effects of phthalates on theca and granulosa cells within the follicle may explain the adverse outcomes associated with phthalate exposure and IVF, such as decreased rates of implantation, clinical pregnancy and live birth. Interestingly, a separate study by Wu et al. (2017) found that male urinary concentrations of phthalate metabolites—but not female—were associated with reduced blastocyst quality in the IVF setting [37].
In contrast, a study by Alur et al. (2015) found that amongst women with a history of infertility, those who conceived after ART had statistically significantly lower first-trimester urinary concentrations of DEHP metabolites compared to those who conceived without ART [35]. The authors suggest that these slightly surprising results may be due to patients undergoing ART pursuing ‘healthier’ lifestyle choices and thereby reducing their exposure to phthalates; the majority of DEHP exposure is through via dietary intake of typically unhealthier, processed food high in animal fat, such as beef, pork and cheese [41, 42].
Overall, the potential impact of phthalates to influence both early and late IVF outcomes cannot be ignored. However, all three studies cited above consist of relatively small sample sizes, reducing the robustness of the results. Further research is needed—ideally systematic reviews or meta-analyses—to provide reliable conclusions regarding the unique actions and effect of the individual phthalate subtypes.
8.2.3 Triclosan
Triclosan is a compound with antimicrobial activity, resulting in widespread use within products such as disinfectants, soaps, deodorants and toothpaste, [15, 23, 24, 43, 44] and also within medical devices [44]. Triclosan has been detected in up to in 98% of urine samples from pregnant women [45] and has also been found in the blood, breast milk and adipose tissue of humans; [24] this is unsurprising given its widespread presence. Its bacteriostatic properties mean triclosan has typically been considered safe for human use; however, emerging evidence suggests that it has the potential to detrimentally impact both thyroid and sex hormone homeostasis [11, 24, 46] leading to debate regarding its necessity in household products [44].
There are few epidemiological studies measuring the relationship between triclosan and ART outcomes. The largest and most recent study found that urinary triclosan concentrations were associated with decreased implantation rate. No effect was seen for the other IVF outcomes assessed, namely: metaphase II oocyte count, quality of embryos, rate of fertilisation and rate of clinical pregnancy [46]. Other human studies assessing early IVF outcomes have differing results: Hua et al. (2017) found triclosan affected top quality embryo and implantation rate [47], whereas Lange et al. (2015) found implantation rate to be unaffected, although oocyte yield was seen to decrease with increased triclosan exposure [48]. In addition, the synergistic effects of triclosan and BPA are thought to influence oocyte development and quality, depending on the time of exposure to the mother [18]. Evidence regarding the effects of triclosan on ART outcomes is therefore scarce and inconclusive, with small sample sizes making results hard to compare. Further research is needed amongst larger, diverse populations to provide further evidence regarding the impact of triclosan on reproductive outcomes.
8.2.4 Parabens
Parabens are a class of EDC used primarily as antimicrobial preservatives within pharmaceuticals, personal care products and food [11, 24, 49]. These chemicals exhibit weak oestrogenic properties, [18, 24, 50] and urine sampling has shown women to have a five-fold higher paraben concentration than men [51]—likely due to their extensive presence in cosmetics [52]. However, despite their widespread presence, there are very few studies investigating the association between parabens and fertility, fecundity and fertility treatment.
Existing data has shown that exposure to mixtures of parabens and phenols can alter ovarian hormone levels, [18] likely through their binding to the oestrogen receptors ER-α and ER-β, [53, 54] potentially impacting any IVF outcomes. One study found an association between increasing urinary propyl paraben levels and decreasing antral follicle and oocyte count in women undergoing IVF treatment, suggesting an association between paraben exposure and diminished ovarian reserve [55]. Further studies have shown an association between urinary methyl and propyl paraben levels and poor embryo quality only, [56] with no statistically significant effects seen upon other IVF outcomes, including oocyte retrieval rate, cleavage rate, implantation, [56] or fertilisation and live birth rates [57].
In summary, data surrounding this topic is scarce. There is an as yet unmet need for further human studies examining the potential effects of parabens on fertility and ART, especially given their abundant presence within the general population. In the meantime, patients undergoing IVF may prefer to minimise their exposure to parabens where possible.
8.2.5 Persistent Organic Pollutants
Persistent organic pollutants (POPs) are a large group of EDCs, with various subgroups including polychlorinated biphenyls (PCBs) and perfluoroalkyl and polyfluoroalkyl substances (PFAS). As suggested by their name, POPs are incredibly stable molecules that resist degradation and are toxic to both humans and animals. This stability results in their bioaccumulation, usually within the adipose tissue of humans and animals [11, 14]. POPs are known to have oestrogenic and anti-androgenic hormone action, impacting both male and female fertility [11, 24, 58]. Prior to a worldwide ban on PCBs in 1979, [12] they were used in fluids for electrical equipment, lubricants and paints [11]. However, these chemicals have long half-lives of up to 5 years, [59] resulting in persistent environmental exposure to these chemicals, [11, 14] primarily through contaminated food (particularly meat, fish and dairy) but also via inhalation and dermal contact with contaminated soil [24]. In contrast, PFAS are still used today in a wide range of products including textiles, pesticides, personal care products and firefighting foams [11, 18, 58, 60].
A recent review of the evidence by Bjorvang et al. (2020) found conflicting evidence in relation to the effect of POPs on IVF outcomes; [14] some studies found no associations, whilst others found the most lipophilic POPs were associated with poorer oocyte quality [61,62,63] and reduced fertilisation rate [62,63,64]. Increasing serum PCB concentration has also been associated with lower rates of high-quality embryos, lower implantation rates and a lower live birth rate in women undergoing IVF treatment [62, 65]. This is in contrast to earlier studies which have suggested chronic exposure to low levels of PCBs does not impact human reproductive [66] or IVF outcomes [67].
The presence of POPs is not restricted to the mother; however, these chemicals are able to cross the placenta, enter foetal circulation and are also present in breast milk [24, 68]. The potential long-term effects of these on the health of the newborn are concerning.
Further experimental and epidemiological studies are clearly needed to clarify the associations seen, which are limited and conflicting. Despite sharing a common chemical structure, each isoform has different biological effects, [69] which limits the extent broad comparisons that can be made across the groups. Efforts should be made to identify exactly which POPs provide the most, if any, risk to human fertility.
8.2.6 Implications for Practice
Studying the impact of EDCs on fertility, fecundity and IVF outcomes is by no means an easy process. Human fertility is dependent on multiple factors, underpinned by the complex underlying mechanisms of processes such as folliculogenesis, spermatogenesis and endometrial receptivity. One of the biggest challenges when examining the effects of EDCs is that these chemicals are vast in number and have varying different biochemical effects—the impact of one cannot be generalised to the impact of them all. Their ubiquitous nature further complicates matters, as these compounds are difficult to avoid and patients will be exposed to many at once. The presence of multiple different chemicals within the human body therefore confounds the effects of any individual chemical studied. For older women, who are more likely to seek ART to conceive due to diminished ovarian reserve, they will experience longer cumulative exposure to these chemicals which may further reduce the chances of pregnancy. Future research needs to focus on the effects of multiple EDCs in combination, as this is more representative of real-life exposure.
Although the literature is growing, particularly examining BPA exposure, for other chemicals such as POPs the evidence is scarce and conflicting. Epidemiological studies have shown associations; however, future studies should be experimental in nature in order to prove causality and mechanisms of action.
Overall, moderate evidence supports associations between increasing EDC concentration, diminishing ovarian reserve and poor IVF outcomes. Women of a reproductive age should therefore minimise exposure to these chemicals where possible, with healthcare professionals actively advocating their avoidance where possible. Clinical practitioners working in reproductive medicine should be educated about the impacts of these endocrine disruptors and be trained to support patients in how to reduce and mitigate their effects. At a population level, social awareness campaigns are needed to advise on the use of consumer products, particularly personal care products, and healthy diets. Arguably, the most important action needed to reduce their impact, however, is urgent worldwide government regulation of EDC use. These measures will help to achieve the ultimate goal of ART treatment for as many patients as possible: the birth of a healthy baby.
8.3 Impact of EDCs on Pregnancy Outcomes
Outside of fertility treatments, there is increasing evidence to suggest EDCs can also impact pregnancies conceived naturally. Maternal exposure to EDCs has been identified as a risk factor for many complications of pregnancy, including recurrent miscarriage, intrauterine growth restriction, gestational hypertensive disorders and pre-term birth [70]. The specific mechanisms by which EDCs cause these adverse effects are unknown; however, it is thought EDCs are able to either accumulate within or act upon the placenta, in order to regulate signalling pathways within trophoblasts resulting in altered cell viability and invasiveness [71]. It is also possible that they trigger an inappropriate immune response in the mother, causing inflammation and oxidative stress [70, 72]. Overall, resultant abnormal placentation can lead to gestational disorders including pre-eclampsia and intrauterine growth restriction, [71, 73] significantly impacting both maternal and foetal health. EDCs can also cause chromosomal abnormalities within oocytes and affect embryo development and implantation, resulting in early pregnancy loss [74].
8.3.1 Miscarriage
Miscarriage—the loss of a pregnancy before 20 weeks gestation—is the most common complication of pregnancy, [75] occurring in 15–25% of all clinically recognised pregnancies [76]. Whilst the majority of sporadic losses are due to chromosomal abnormalities in the foetus, other factors including maternal anatomy, endocrine system and psychological status are also known to influence viability. Environmental exposure is another important factor; it is thought that exposure to air pollutants, including carbon monoxide and particulate matter, is associated with an increased risk of both miscarriage and stillbirth [77]. A similar pattern of evidence is emerging regarding exposure to EDCs. One of the earliest studies in this area found that maternal exposure to BPA was associated with recurrent miscarriage [78]. Laboratory models have confirmed this, with showing that BPA increases the risk of miscarriage at the level of both the endometrium and the oocyte, [75] by affecting the differentiation of uterine stromal to decidual cells [79]. Furthermore, certain phthalate metabolites have also been associated with higher rates of miscarriage, [80, 81] as have the PFAS perfluorooctanoic acid (PFOA) and perfluoroheptane sulfonate (PFHpS) [82]. In contrast, other EDCs including PCBs have not been associated with pregnancy loss, in patients with a history of recurrent miscarriage [83]. However, it is important to recognise that the evidence for all EDCs under consideration is poor; largely comprising of small studies with weak statistical power. Larger epidemiological studies are needed to confirm the effects seen, alongside mechanistic studies in order to understand the mechanism behind the EDCs, this may then allow for the development of therapeutics to mitigate their effects, enabling mothers to have a better chance of a viable pregnancy.
8.3.2 Preeclampsia
Hypertensive disorders of pregnancy, including preeclampsia, pose a significant risk to the health of both mother and foetus; an estimated 10–15% of all maternal deaths are associated with preeclampsia and eclampsia [84]. This complex disease is associated with improper placentation and remodelling of the uterine spiral arteries [85], although the pathophysiology is not completely understood. Evidence is emerging regarding exposure to environmental toxicants as risk factors for the development of gestational hypertensive disorders. Strong evidence already exists to show significant associations between maternal exposure to chemicals including cadmium and lead and the development of preeclampsia [84, 86, 87]. In contrast, the evidence in regard to endocrine disruptors is scarce and conflicting. Six PFAS subtypes have shown positive associations with hypertensive disorder development in several studies, but not all [88]. Inconclusive results have also been found for BPA and phthalates [75, 87, 89]. However, mechanistic studies have shown EDCs such as BPA and PFAS are able to cause abnormal trophoblast invasion, impaired placental function and placental ischaemia [71, 90]. Given what is understood about the aetiology of preeclampsia, it would fit that EDCs may be able to mediate increased incidence of this disease; it is therefore imperative that future research into this area is prioritised.
8.3.3 Infant Birth Weight
Birth size acts as a significant predictor for future health. A low birth weight is associated with increased development of obesity, cardiovascular disease, and non-insulin-dependent diabetes in later life, [91, 92] and these infants are also more likely to need immediate healthcare in the days following birth. Conflicting evidence exists regarding the impact endocrine disruptors might have on infant birth weight. For example, maternal urinary concentration of several different phenols has been associated with decreased birth weight in male infants, with no such association seen for phthalate metabolites [93, 94]. In contrast, BPA alone has been associated with increased head circumference in the same population [93]. In female infants, antenatal exposure to PFAS has been identified as the EDC class to have the strongest association with reduced birth weight, [91] though several other studies have failed to find significant associations for other EDCs [95, 96]. A recent systematic review examining the role of BPA and foetal growth velocity found mounting evidence to suggest that BPA exposure leads to foetal growth restriction and reduced birth size, particularly when exposure occurs in the first half of pregnancy [97]. Additionally, exposure to EDCs including phthalates and PFOS have been associated with a significantly increased chance of premature delivery [98, 99], which is likely to lead to reduced birth weight and is an independent cause of neonatal mortality. Despite the contradictory evidence, as with the previous outcomes discussed, it should be recommended that exposure to endocrine disruptors is as minimal as possible during pregnancy. This reduction in exposure and hopeful subsequent reduction in incidence of low birth weight would have a significant public health impact, given its downstream consequences.
8.3.4 Cholestasis
There is very little evidence pertaining to associations between EDCs and intra-hepatic cholestasis of pregnancy (ICP). ICP causes reversible liver dysfunction from the third trimester of pregnancy until delivery in between 0.5% and 1.8% of pregnancies in Europe [100]. It is characterised by elevated serum bile acid and transaminase levels, with resultant maternal pruritis, particularly on the palms and soles. There is a little evidence to suggest that maternal exposure to EDCs acts as a risk factor for the development of ICP. For example, a recent cohort study found an association between higher urine levels of certain phthalates in the first trimester and increased incidence of ICP [101]. Further research is clearly needed to determine the impact exposure to EDCs may have on the development of ICP, and by what mechanism.
8.4 Conclusion
It is clear that endocrine-disrupting chemicals pose significant risk to the reproductive health of both males and females, with potential downstream effects lasting for years to come. Although evidence is frequently conflicting, it is undeniable that public health is significantly threatened by these offending chemicals. Identifying the risk of a single EDC is challenging and relatively meaningless, as we are exposed to many hundreds of these substances from conception. Further research is therefore needed to examine the impact of different mixtures of EDCs in combination and to understand their mechanisms of action. In the meantime, a strategy is urgently needed to prevent the exposure to these chemicals, especially in women of child-bearing age, to mitigate their impact on the outcomes of pregnancy and assisted reproduction. This will not only improve public health but ensure as many parents as possible achieve reproductive success with the birth of healthy babies.
References
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Conforti A, Mascia M, Cioffi G, De Angelis C, Coppola G, De Rosa P, et al. Air pollution and female fertility: a systematic review of literature. Reprod Biol Endocrinol. 2018;16(1):117.
Yuan M, Bai M-Z, Huang X-F, Zhang Y, Liu J, Hu M-H, et al. Preimplantation exposure to Bisphenol A and Triclosan may Lead to implantation failure in humans. Biomed Res Int. 2015;2015:1.
Ravitsky V, Kimmins S. The forgotten men: rising rates of male infertility urgently require new approaches for its prevention, diagnosis and treatment. Biol Reprod. 2019;101(5):872–4.
Canipari R, De Santis L, Cecconi S. Female fertility and environmental pollution. Int J Environ Res Public Health. 2020;17(23):8802.
Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32(1):18–31.
Li Q, Zheng D, Wang Y, Li R, Wu H, Xu S, et al. Association between exposure to airborne particulate matter less than 2.5 μm and human fecundity in China. Environ Int. 2021;146:106231.
Altmäe S, Haller K, Peters M, Hovatta O, Stavreus-Evers A, Karro H, et al. Allelic estrogen receptor 1 (ESR1) gene variants predict the outcome of ovarian stimulation in in vitro fertilization. Mol Hum Reprod. 2007;13(8):521–6.
Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):498–506.
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342.
Green MP, Harvey AJ, Finger BJ, Tarulli GA. Endocrine disrupting chemicals: impacts on human fertility and fecundity during the peri-conception period. Environ Res. 2021;194:110694.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150.
Yilmaz B, Terekeci H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. 2020;21(1):127–47.
Björvang RD, Damdimopoulou P. Persistent environmental endocrine-disrupting chemicals in ovarian follicular fluid and in vitro fertilization treatment outcome in women. Ups J Med Sci. 2020;125(2):85–94.
Mínguez-Alarcón L, Gaskins AJ. Female exposure to endocrine disrupting chemicals and fecundity: a review. Curr Opin Obstet Gynecol. 2017;29(4):202–11.
Dominguez F. Phthalates and other endocrine-disrupting chemicals: the 21st century's plague for reproductive health. Fertil Steril. 2019;111(5):885–6.
Neel BA, Sargis RM. The paradox of Progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60(7):1838.
Xu X, Yang M. Effects of environmental EDCs on oocyte quality, embryo development, and the outcome in human IVF process. Adv Exp Med Biol. 2021;1300:181–202.
Sharma A, Mollier J, Brocklesby RWK, Caves C, Jayasena CN, Minhas S. Endocrine-disrupting chemicals and male reproductive health. Reprod Med Biol. 2020;19(3):243–53.
Eskew AM, Jungheim ES. A history of developments to improve in vitro fertilization. Mo Med. 2017;114(3):156–9.
Stephen EH, Chandra A, King RB. Supply of and demand for assisted reproductive technologies in the United States: clinic- and population-based data, 1995-2010. Fertil Steril. 2016;105(2):451–8.
Human Fertilisation and Embryology Authority Fertility treatment 2019: trends and figures. 2021. hfea.gov.uk.
Chiang C, Mahalingam S, Flaws JA. Environmental contaminants affecting fertility and somatic health. Semin Reprod Med. 2017;35(3):241–9.
Karwacka A, Zamkowska D, Radwan M, Jurewicz J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence. Hum Fertil (Camb). 2019;22(1):2–25.
Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33(2):385–93.
Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016). Fertil Steril. 2016;106(4):827–56.
Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122(8):775–86.
Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70.
Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol a concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27(12):3583–92.
Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120(7):978–83.
Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Kaleta D, et al. Urinary bisphenol A concentrations and in vitro fertilization outcomes among women from a fertility clinic. Reprod Toxicol. 2020;96:216–20.
Machtinger R, Orvieto R, Bisphenol A. Oocyte maturation, implantation, and IVF outcome: review of animal and human data. Reprod BioMed Online. 2014;29(4):404–10.
Cao Y, Qu X, Ming Z, Yao Y, Zhang Y. The correlation between exposure to BPA and the decrease of the ovarian reserve. Int J Clin Exp Pathol. 2018;11(7):3375–82.
Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283(1):12–8.
Alur S, Wang H, Hoeger K, Swan SH, Sathyanarayana S, Redmon BJ, et al. Urinary phthalate metabolite concentrations in relation to history of infertility and use of assisted reproductive technology. Fertil Steril. 2015;104(5):1227–35.
Du Y, Guo N, Wang Y, Teng X, Hua X, Deng T, et al. Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertil Steril. 2019;111(5):953–61.
Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, et al. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod. 2017;32(1):65–75.
Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab. 2014;99(11):4346–52.
Paoli D, Pallotti F, Dima AP, Albani E, Alviggi C, Causio F, et al. Phthalates and Bisphenol A: presence in blood serum and follicular fluid of Italian women undergoing assisted reproduction techniques. Toxics. 2020;8(4):91.
Pallotti F, Pelloni M, Gianfrilli D, Lenzi A, Lombardo F, Paoli D. Mechanisms of testicular disruption from exposure to Bisphenol a and Phtalates. J Clin Med. 2020;9(2):471.
Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43.
Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol a and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914–20.
Chen M, Tang R, Fu G, Xu B, Zhu P, Qiao S, et al. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater. 2013;250–251:115–21.
Yueh M-F, Tukey RH. Triclosan: a widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol. 2016;56(1):251–72.
Wang X, Ouyang F, Feng L, Liu Z, Zhang J. Maternal urinary Triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective study. Environ Health Perspect. 2017;125(6):067017.
Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Zajdel R, et al. Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environ Sci Pollut Res. 2021;28(10):12993–9.
Hua R, Zhou Y, Wu B, Huang Z, Zhu Y, Song Y, et al. Urinary triclosan concentrations and early outcomes of in vitro fertilization-embryo transfer. Reproduction. 2017;153(3):319–25.
Lange A, Carignan CC, Minguez-Alarcon L, Williams P, Calafat AM, Toth TL, et al. Triclosan exposure and treatment outcomes in women undergoing in vitro fertilization. Fertil Steril. 2015;104(3):e86.
Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–66.
Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility—is there a mitochondrial connection? Reprod Toxicol. 2009;27(1):1–7.
Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120(11):1538–43.
Cabaleiro N, de la Calle I, Bendicho C, Lavilla I. An overview of sample preparation for the determination of parabens in cosmetics. TrAC Trends Anal Chem. 2014;57:34–46.
Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, et al. Estrogenic activity of cosmetic components in reporter cell lines: parabens, UV screens, and musks. J Toxicol Environ Health A. 2005;68(4):239–51.
Nowak K, Ratajczak-Wrona W, Górska M, Jabłońska E. Parabens and their effects on the endocrine system. Mol Cell Endocrinol. 2018;474:238–51.
Smith KW, Souter I, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ Health Perspect. 2013;121(11–12):1299–305.
Sabatini ME, Smith KW, Ford J, Ehrlich SR, Toth TL, Hauser R. Urinary paraben concentrations and in vitro fertilization (IVF) outcomes. Fertil Steril. 2011;96(3):S154.
Mínguez-Alarcón L, Chiu YH, Messerlian C, Williams PL, Sabatini ME, Toth TL, et al. Urinary paraben concentrations and in vitro fertilization outcomes among women from a fertility clinic. Fertil Steril. 2016;105(3):714–21.
Ma X, Cui L, Chen L, Zhang J, Zhang X, Kang Q, et al. Parental plasma concentrations of perfluoroalkyl substances and in vitro fertilization outcomes. Environ Pollut. 2021;269:116159.
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–305.
McCoy JA, Bangma JT, Reiner JL, Bowden JA, Schnorr J, Slowey M, et al. Associations between perfluorinated alkyl acids in blood and ovarian follicular fluid and ovarian function in women undergoing assisted reproductive treatment. Sci Total Environ. 2017;605–606:9–17.
Younglai EV, Foster WG, Hughes EG, Trim K, Jarrell JF. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol. 2002;43(1):121–6.
Petro EM, Leroy JL, Covaci A, Fransen E, De Neubourg D, Dirtu AC, et al. Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod. 2012;27(4):1025–33.
Bloom MS, Fujimoto VY, Storm R, Zhang L, Butts CD, Sollohub D, et al. Persistent organic pollutants (POPs) in human follicular fluid and in vitro fertilization outcomes, a pilot study. Reprod Toxicol. 2017;67:165–73.
Al-Hussaini TK, Abdelaleem AA, Elnashar I, Shabaan OM, Mostafa R, El-Baz MAH, et al. The effect of follicullar fluid pesticides and polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological and clinical outcome. Eur J Obstet Gynecol Reprod Biol. 2018;220:39–43.
Meeker John D, Maity A, Missmer Stacey A, Williams Paige L, Mahalingaiah S, Ehrlich S, et al. Serum concentrations of polychlorinated biphenyls in relation to in vitro fertilization outcomes. Environ Health Perspect. 2011;119(7):1010–6.
Khanjani N, Sim MR. Maternal contamination with PCBs and reproductive outcomes in an Australian population. J Expo Sci Environ Epidemiol. 2007;17(2):191–5.
Jirsová S, Masata J, Jech L, Zvárová J. Effect of polychlorinated biphenyls (PCBs) and 1,1,1-trichloro-2,2,-bis (4-chlorophenyl)-ethane (DDT) in follicular fluid on the results of in vitro fertilization-embryo transfer (IVF-ET) programs. Fertil Steril. 2010;93(6):1831–6.
Müller MHB, Polder A, Brynildsrud OB, Grønnestad R, Karimi M, Lie E, et al. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ Res. 2019;170:433–42.
Pocar P, Brevini TA, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction. 2003;125(3):313–25.
Schjenken JE, Green ES, Overduin TS, Mah CY, Russell DL, Robertson SA. Endocrine disruptor compounds-a cause of impaired immune tolerance driving inflammatory disorders of pregnancy? Front Endocrinol. 2021;12:607539.
Yang C, Song G, Lim W. A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere. 2019;231:326–36.
Rogers JA, Metz L, Yong VW. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53(4):421–30.
Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-a in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36(6):699–703.
Liu Q. Effects of environmental endocrine-disrupting chemicals on female reproductive health. Adv Exp Med Biol. 2021;1300:205–29.
Krieg SA, Shahine LK, Lathi RB. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril. 2016;106(4):941–7.
The Practice Committee Of The American Society For Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11.
Grippo A, Zhang J, Chu L, Guo Y, Qiao L, Myneni AA, et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health. 2018;33(3):247–64.
Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–9.
Li Q, Davila J, Bagchi MK, Bagchi IC. Chronic exposure to bisphenol A impairs progesterone receptor-mediated signaling in the uterus during early pregnancy. Receptors. Clin Investig. 2016;3(3):e1369.
Mu D, Gao F, Fan Z, Shen H, Peng H, Hu J. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ Sci Technol. 2015;49(17):10651–7.
Toft G, Jönsson BAG, Lindh CH, Jensen TK, Hjollund NH, Vested A, et al. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect. 2012;120(3):458–63.
Liew Z, Luo J, Nohr EA, Bech BH, Bossi R, Arah OA, et al. Maternal plasma Perfluoroalkyl substances and miscarriage: a nested case-control study in the Danish National Birth Cohort. Environ Health Perspect. 2020;128(4):47007.
Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. PCBs, hexachlorobenzene and DDE are not associated with recurrent miscarriage. Am J Reprod Immunol. 2003;50(6):485–9.
Rosen EM, Muñoz MI, McElrath T, Cantonwine DE, Ferguson KK. Environmental contaminants and preeclampsia: a systematic literature review. J Toxicol Environ Health B Crit Rev. 2018;21(5):291–319.
Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–58.
Poropat AE, Laidlaw MAS, Lanphear B, Ball A, Mielke HW. Blood lead and preeclampsia: a meta-analysis and review of implications. Environ Res. 2018;160:12–9.
Gómez-Roig MD, Pascal R, Cahuana MJ, García-Algar O, Sebastiani G, Andreu-Fernández V, et al. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn Ther. 2021;48(4):245–57.
Erinc A, Davis MB, Padmanabhan V, Langen E, Goodrich JM. Considering environmental exposures to per- and polyfluoroalkyl substances (PFAS) as risk factors for hypertensive disorders of pregnancy. Environ Res. 2021;197:111113.
Pergialiotis V, Kotrogianni P, Christopoulos-Timogiannakis E, Koutaki D, Daskalakis G, Papantoniou N. Bisphenol A and adverse pregnancy outcomes: a systematic review of the literature. J Matern Fetal Neonatal Med. 2018;31(24):3320–7.
Marinello WP, Mohseni ZS, Cunningham SJ, Crute C, Huang R, Zhang JJ, et al. Perfluorobutane sulfonate exposure disrupted human placental cytotrophoblast cell proliferation and invasion involving in dysregulating preeclampsia related genes. FASEB J. 2020;34(11):14182–99.
Marks KJ, Howards PP, Smarr MM, Flanders WD, Northstone K, Daniel JH, et al. Prenatal exposure to mixtures of persistent endocrine-disrupting chemicals and birth size in a population-based cohort of British girls. Epidemiology. 2021;32(4):573–82.
Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33.
Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120(3):464–70.
Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–7.
Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds in relation to birth weight in the Norwegian mother and child cohort study. Am J Epidemiol. 2012;175(12):1209–16.
Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16(5):641–7.
Vrachnis N, Loukas N, Vrachnis D, Antonakopoulos N, Zygouris D, Kοlialexi A, et al. A systematic review of Bisphenol A from dietary and non-dietary sources during pregnancy and its possible connection with fetal growth restriction: investigating its potential effects and the window of fetal vulnerability. Nutrients. 2021;13(7):2426.
Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7.
Deji Z, Liu P, Wang X, Zhang X, Luo Y, Huang Z. Association between maternal exposure to perfluoroalkyl and polyfluoroalkyl substances and risks of adverse pregnancy outcomes: a systematic review and meta-analysis. Sci Total Environ. 2021;783:146984.
Floreani A, Caroli D, Lazzari R, Memmo A, Vidali E, Colavito D, et al. Intrahepatic cholestasis of pregnancy: new insights into its pathogenesis. J Matern Fetal Neonatal Med. 2013;26(14):1410–5.
Wang JQ, Gao H, Sheng J, Tao XY, Huang K, Zhang YW, et al. Urinary concentrations of phthalate metabolites during gestation and intrahepatic cholestasis of pregnancy: a population-based birth cohort study. Environ Sci Pollut Res Int. 2020;27(11):11714–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
D’Angelo, A., St Pier, G. (2023). Endocrine-Disrupting Chemicals (EDCs) and Reproductive Outcomes. In: Marci, R. (eds) Environment Impact on Reproductive Health. Springer, Cham. https://doi.org/10.1007/978-3-031-36494-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-36494-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36493-8
Online ISBN: 978-3-031-36494-5
eBook Packages: MedicineMedicine (R0)