Abstract
A growing number of scientific studies have shown, since the last decade, increasing evidence suggesting that the human health and wildlife could be affected by a wide range of substances broadly disseminated in the environment and also found recurrently in a wide array of everyday products. These products were identified as toxicants with various effects on endocrine processes and functions as neoplasm development, reproductive dysfunctions, and immunological and thyroid disorders [1]. These endocrine-disrupting chemicals (EDCs), which are defined as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” [2], are not rogue pharmaceuticals or rare contaminants.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
5.1 Introduction
A growing number of scientific studies have shown, since the last decade, increasing evidence suggesting that the human health and wildlife could be affected by a wide range of substances broadly disseminated in the environment and also found recurrently in a wide array of everyday products. These products were identified as toxicants with various effects on endocrine processes and functions as neoplasm development, reproductive dysfunctions, and immunological and thyroid disorders [1]. These endocrine-disrupting chemicals (EDCs), which are defined as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” [2], are not rogue pharmaceuticals or rare contaminants.
EDCs enter the human body via food, water, dust by inhalation, and the transdermal route after contact and using cosmetics and creams. Transplacental transfer of these substances to the developing fetus has also been demonstrated [3] and therefore can be found in all body fluids (urine, serum, breast milk, and amniotic fluid). EDCs can accumulate and alter the adipose tissue, pancreas, liver, gastrointestinal tract, muscle, and brain homeostatic and hedonic pathways [4], and the effects of their metabolites can be functional at low doses and can persist for a long time [5].
The US Food and Drug Administration identified more than 1800 chemicals that disrupt at least one of the three endocrine pathways (estrogen, androgen, and thyroid) [6], and 320 of 575 chemicals were screened during the instruction of the European Commission, with either evidence or potential evidence for endocrine disruption. Today, medical societies and governmental agencies such as the Endocrine Society [7], the International Federation of Gynecology and Obstetrics [8], the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) [9], and the American Academy of Pediatrics [10] document the rapidly accelerating evidence and implications for human health. EDCs are usually used by the industries, as plastics (bisphenol A [BPA]), plasticizers (phthalates), solvents/lubricants (polybrominated biphenyls [PBBs], polychlorinated biphenyls [PCBs], and dioxins), pesticides (chlorpyrifos, dichlorodiphenyltrichloroethane [DDT], and methoxychlor), fungicides (vinclozolin), and also as flame-retardant additives in manufactured materials and pharmaceutical agents, for example, diethylstilbestrol (DES), a nonsteroidal synthetic estrogen [11]. EDCs may also be made by nature; for example, phytoestrogens, which interfere with endogenous endocrine function, are produced by plants and act primarily through estrogen receptors [12].
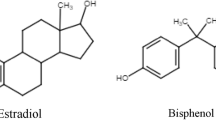
One of the most widely discussed and abundant EDCs is bisphenol A (BPA). Bisphenols are found in polycarbonates, epoxy resins, food, cosmetics packaging, and even dental composite materials [13]. BPA is able to interact with estrogen receptors through its phenolic structure: This allows the modification of hormonal homeostasis via a combination of agonist and/or antagonist actions depending on the target tissue. BPA does act not only on estrogens but also on androgen, pregnane X, thyroid, and glucocorticoid receptors [14]. Although in the recent years the use of BPA has been limited, and replaced in some products by its structural analogs such as bisphenol S (BPS), bisphenol F (BPF), and bisphenol AF (BPAF), comparable endocrine-disrupting effects have been observed with these alternative bisphenols, as the metabolism and mechanism of action are similar to BPA [15]. Unfortunately, BPS are not the only EDCs, and our body is subject to a “cocktail effect” as the addition and multiplication of each EDC occurs and can amplify the risks. Many personal care products, foods, and pharmaceuticals contain mixtures of bisphenols, parabens, and other EDCs; esters of p-hydroxybenzoic acid are used as antimicrobial agents and preservatives. In addition to the estrogenic effect, several parabens also possess antiandrogenic activity as they can bind to androgen receptors and thereby inhibit testosterone-induced transcription [16]. Methylparaben (MP) and propylparaben (PP), along with ethylparaben (EP), butylparaben (BP), and benzylparaben (benzylP), are among the most commonly used. In vivo studies indicate that parabens can disrupt reproduction, development, and homeostasis. In humans, they have been detected in serum, urinary cord blood, meconium, milk, amniotic fluid, and placental tissue [17, 18]. Relevant associations of MP and hormones affecting metabolic health and energy were observed, indicating obesogenic potential. Associations of methylparaben and hormones affecting energy balance and metabolic health have been observed, indicating its obesogenic potential [19]. Moreover, their effect seems to be transgenerational, occurring over at least two or three generations. As the window of susceptibility, puberty is considered as the one of the hot spots in the lifetime when EDCs may exert their effects [20], and areas of concern appear to be conditions like polycystic ovaries pathology [21, 22], precocious puberty issues [23], and endometriosis [24,25,26,27].
5.2 PCOS
Polycystic ovary syndrome (PCOS) is a complex and heterogeneous endocrine disorder in women of reproductive age [28]. Its prevalence is estimated to be between 5% and 10% and even up to 21%, depending on the diagnostic criteria and the geographic location [29,30,31]. In 1990, the National Institute of Health proposed the following diagnostic criteria: the presence of clinical and/or biochemical hyperandrogenism and oligomenorrhea/amenorrhea with anovulation [32]. In 2006, the Androgen Excess Society proposed diagnostic criteria: an androgen in excess is a critical element in the development and pathogenesis of PCOS that should be present and accompanied by oligomenorrhea, polycystic ovarian morphology, or both [30]. According to the Rotterdam criteria, the PCOS diagnosis requires meeting two of the three criteria mentioned above [33]. Today, the Rotterdam criteria are used by the medicals professional and researchers [34].
Hyperandrogenism seems to be the key feature of PCOS that contributes to clinical phenotypes and fertility dysregulation [35]. The most common sequelae of hyperandrogenism in the setting of the PCOS phenotype are hirsutism, acne, and alopecia [36]. The hormonal and metabolic alterations may result in reproductive disruption, including menstrual cycle dysfunction, chronic anovulation, and infertility [37], and the majority of women with PCOS have insulin resistance [38, 39], which may lead to the development of obesity [40]. This obesity is characterized by metabolic disturbances similar to metabolic syndrome [41] such atherogenic dyslipidemia and decreased glucose tolerance, which can lead to type 2 diabetes [42], with higher blood pressure values, increased thrombotic activity and several cardiovascular markers [43], and hyperinsulinemia and peripheral insulin resistance, which can occur independently on body weight [44]. Obesity, which is not always found in the ovaries with PCOS, where insulin resistance and compensatory hyperinsulinemia seem to play a vital role in the mechanisms of reproductive disorders by directly affecting the insulin-resistant ovaries with PCOS, has a detrimental impact on the ovulation process [21]. Conceiving difficulties may be due to slightly enlarged ovaries with numerous antral follicles, by two- to three-fold that of normal ovaries causing irregular ovulation and oligomenorrhea/amenorrhea. Other several features of PCOS are an excess of androgen [45], with a correlation with of a two- to three-fold higher anti-Müllerian hormone (AMH) than in ovulatory women with normal ovaries [46].
In addition, a “vicious circle” of hyperandrogenemia is created [47], following an elevated luteinizing hormone (LH) levels that promote androgen production and a reduction in estrogens. The underlying causes of PCOS are unclear, likely both genetic and environmental/nutritional, and the variety of clinical manifestations raises the possibility that multiple etiological factors simultaneously promote the final PCOS phenotype [28]. While geographic location, ethnicity, lifestyle, and environmental factors [48] appear to play a role, the latter along with endocrine-disrupting chemicals (EDCs) in the pathogenetic mechanisms of PCOS has been evoked recently. EDCs are a heterogeneous group of molecules, of natural or synthetic origin, capable of interacting with the endocrine system [28] by affecting hormonal biosynthesis, modifying their genomic and nongenomic effects, modifying the mechanisms of control and regulation and their epigenetic manifestations [18]. EDCs can be found in many everyday products (e.g., plastic bottles, cosmetics, metal cans, flame retardants, detergents, foods, toys, and pesticides) and penetrate in an organism through the ingestion of contaminated food and liquids, the breathing of contaminated air, and transdermal absorption [49]. Although in the recent years the use of BPA has been limited, and replaced with some products by its structural analogs such as bisphenol S (BPS), bisphenol F (BPF), and bisphenol AF (BPAF), comparable endocrine-disrupting effects have been observed with these alternative bisphenols, as the metabolism and mechanism of action are similar to BPA [15, 50]. The serum concentration of BPA is elevated in PCOS and correlates with androgen levels [51, 52]. The data suggest that reproductive function is disturbed directly at the ovary level by affecting ovarian steroid hormone production and the maturation of the follicle or indirectly by interfering with the hypothalamic–pituitary axis [21]. As obesity is associated with PCOS, low-grade inflammation, and increased inflammatory cytokines, several groups have indicated elevated levels of specific cytokines in women with PCOS, pointing out that chronic low-grade inflammation may affect the development of ovarian dysfunction and metabolic derangement [53, 54] The question is if the principal role in low-grade inflammation is due to only obesity or also due to PCOS. It is known from the literature that the interaction between BPA and testosterone is complex. On the one hand, testosterone interacts with BPA metabolism by decreasing uridine diphosphate glucuronosyl transferase activity, which leads to increased levels of BPA. On the other hand, BPA interferes with testosterone metabolism first by the inhibition of testosterone hydroxylases (2- and 6-hydroxylase), which are not that important in the degradation of testosterone as much as oxidoreductases, but still can play a role in its metabolism, and secondary by displacing testosterone on sex hormone–binding globulin (SHBG), which leads to the increase of circulating free androgen concentration [21].These interactions, especially the influence on binding protein, could explain our findings of the correlation between BPA exposure and testosterone only in a healthy control group, unlike in PCOS women, where the testosterone levels are high; thus, a “vicious circle” with BPA is formed.
The higher levels of BPA in PCOS patients were found compared with healthy controls [51, 52] without differences between those with normal-weight and obese ones and higher cytokines levels in obese ones with PCOS [13], which, in complexity, reflect activation and proinflammatory state. Findings in obese women with PCOS (insulin resistance, lousy lipid profile, risk of fatty liver disease, and proinflammatory state) compared with normal-weight PCOS women, which have very similar metabolic profile as healthy control, are confirmation of how obesity could obscure the searching of PCOS etiopathogenesis. The combination of genetic predispositions associated with environmental factors favored PCOS. In this context, being able to interact with the metabolism of testosterone, EDCs constitute one of the causes of PCOS.
We can conclude that these findings confirm that BPA could be one of the essential elements in the PCOS etiopathogenesis [13].
It is important to emphasize that other studies will have to be done because the number of endocrine disruptors continues to increase. For women with PCOS, it is essential that they maintain their body weight within normal range as this may protect them from the metabolic complications associated with this condition.
5.3 Early Puberty and EDCs
Early puberty is defined by the presence of clinical and auxological signs of pubertal development between the age of 8 and 10 years [55], between the age of 7.5 and 8.5 years [56], or between the age of 8 and 9 years [57]. Some authors consider that, when pubertal onset occurs before the age of 8 years, it is considered precocious, and when it occurs after 8 years but before 9 years of age, it is considered early. The mechanism of early pubertal development has not been clarified yet [58]. Puberty begins with the release of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator from central nervous system inhibition after a quiescent period during childhood [59]. The age of menarche has definitely decreased from 16 years in the 1800s to 13 years in the 1960s, after which this downward trend seems to have slowed or even stopped [60]. Although genetic factors remain the main determinant of the timing of puberty [61], the trend toward earlier onset of puberty has coincided with improvements in public health and nutrition [62]. At the same time, endocrine-disrupting chemicals (EDCs) have been suggested as affecting the age of pubertal onset, especially in girls. Hence, researchers were led to hypothesize that increasing exposure to EDC had a role in the trend for earlier sexual maturation. Moreover, it was suggested that early puberty manifesting in immigrants from the developing countries was the result of previous exposure to organochlorine pesticides [63]. Constitutional advancement of growth (CAG) is the growth pattern of early growth acceleration, which is present in the majority of girls with idiopathic precocious puberty and in girls with early puberty [64]. While endocrine disruptors are commonly used by the industries, such as plastics (bisphenol A [BPA]), solvents/lubricants (polybrominated biphenyls [PBBs], polychlorinated biphenyls [PCBs]), dioxins, plasticizers (phthalates), pesticides (chlorpyrifos, dichlorodiphenyltrichloroethane [DDT], and methoxychlor), fungicides (vinclozolin), flame-retardant additives in manufactured materials and pharmaceutical agents, for example, diethylstilbestrol (DES), a nonsteroidal synthetic estrogen [11], they can also be natural, for example, phytoestrogens, produced by plants and that act mainly through estrogen receptors [12]. The large quantity of endocrine disruptors and their ability to interact with the endocrine system combined with the tendency toward the early onset of puberty have led many researchers to associate them with precocious puberty, especially since they have estrogenic activity. Several EDCs have been studied, and we will cite the main ones such as phthalates, bisphenol A (BPA), pesticides, flame-retardant chemicals, and PCBs.
5.4 Phthalates
Phthalates are esters of phthalic anhydride, used as liquid plasticizers in plastics, flooring, personal care products, medical devices, and tubing because they increase the flexibility, transparency, durability, and longevity of materials. Their most common use is to soften polyvinyl chloride (PVC). Phthalates can be classified as low- or high-molecular-weight phthalates, and depending on the class and the timing of exposure, different outcomes have been observed.
Their endocrine-disrupting mechanism is not fully clarified, but they act either as estrogen receptor agonists and antagonists or as androgen receptor antagonists and can also disrupt androgen synthesis. Different studies have demonstrated a significant association with premature thelarche and precocious or early puberty [65, 66]. High-molecular-weight phthalate levels several years before puberty are associated with later pubic hair development and younger age of menarche. Low-molecular-weight phthalate levels are related to advanced breast or pubic hair development [67]. In a study of the Danish schoolgirls, high phthalate excretion in urine was associated with delayed pubarche, but not thelarche, which suggests antiandrogenic actions of phthalate [68]. Similar results were obtained in a study of the US girls [69]. In contrast, in another study on the US girls with central precocious puberty (CPP), such an association was not found [70], and furthermore, a recent Korean study showed that phthalate metabolites in girls with central precocious puberty were significantly lower than the prepubertal control girls [71]. Many results are conflicting, and further studies are needed to confirm or refute the effect of phthalate exposure on pubertal timing.
5.5 BPA (Bisphenol A)
BPA is a precursor of plastics, polycarbonates, and epoxy resins coating the inside of beverage, found in plastics (e.g., bottles, Tupperware, food cans, etc.). It is the most commonly found estrogen-like endocrine disruptor that can also act as an antiandrogen in the environment. This chemical is almost ubiquitous, and even if the estrogen receptor agonist activity is weak, its potential should not be underestimated. In some experimental animals, it has been shown that BPA advances puberty [72], but no effect on pubertal timing [73]. Similar to the experimental animals, the results of BPA on human puberty are inconsistent. In a study of the US girls, Wolff et al. reported that BPA had no influence on breast development [67]; however, in studies performed in Turkey and in Thailand, idiopathic central precocious puberty was associated with higher levels of BPA than in control girls [74, 75]. Watkins et al. studied the in utero and peripubertal exposure to phthalates and BPA in relation to sexual maturation and did not find any association between BPA and sexual maturation, although in utero phthalate exposure impacted on earlier timing of sexual maturation [76]. Other studies shown that EDCs are associated with premature thelarche, precocious puberty, and pubertal development [74, 77, 78]. On the other hand, in a recent review, of 19 studies, only seven showed a correlation between BPA and puberty with evidence of the possible disruptive role of BPA in people with central precocious puberty or isolated premature breast development aged from 2 months to 4 years, although the mechanism is not defined. Some studies have also found a close relationship between urinary BPA, body weight, and precocious puberty, which may be explained by the obesogenic effect of BPA itself [79].
5.6 Pesticides
They are classified into various classes, for example, insecticides, herbicides, and fungicides, and can enter the human body through water, air, and food and can pass from mother to fetus via the placenta and to the infant through mother’s milk. One of the well-known dichlorodiphenyltrichloroethane (DDT) is an organochlorine, originally developed as an insecticide for use in agriculture. Exposure to DDT is imperceptible, because it is odorless, tasteless, and colorless, and being exposed during fetal life and lactation can affect sexual development. Despite the fact that DDT is still widely used in some low-income countries and has been banned from our markets, it can persist in the environment as a persistent organic pollutant (POP). Dichlorodiphenyldichloroethane (DDE), a metabolite of DDT, has antiandrogenic, antiprogestin, and estrogenic effect and induces aromatase. Vasiliu et al. found an association between the exposure to these chemicals and precocious puberty and earlier age of menarche [80].
A study performed in Denmark, female offspring of mothers exposed to pesticide in a greenhouse showed a decreased age of breast development at 8.9 years, compared with 10.4 years in the unexposed population and 10.0 years in a Danish reference population [81], but the significance of the association disappeared when weight at menarche was controlled for. Pesticide exposure to pesticides has also been suggested in adopted or immigrant girls in Belgium, with central precocious puberty (CPP), following the discovery of higher levels of plasma DDE [63]. Conversely, other studies did not found an association between DDE levels and early puberty [82], but unlike a puberty delay [83].
Flame-retardant chemicals are added to the manufactured materials (plastics, textiles, surface finishes, and coatings) intended to prevent or slow the further development of ignition with their physical and chemical properties. Among them, organohalogen compounds such as polybrominated diphenyl ethers (PBDEs) are lipophilic persistent endocrine disruptors exhibiting estrogenic and androgenic properties. PBDEs might alter pubertal timing, resulting in later menarche in girls [84], but in girls with idiopathic central precocious puberty, particularly those with higher body mass index (BMI) have been found with higher serum concentrations of PBDEs [85]. Thus, the inconsistency of the results of the various studies examining the association of endocrine disruptor chemicals with the onset of puberty [86] makes it imperative that more studies on the subject are performed.
Polychlorinated biphenyl (PCB) is a dioxin-like compound derived from biphenyl, used as a dielectric and coolant fluid in electrical apparatuses. Its mechanism of action is rather similar to that of dioxins, and there is evidence that exposure during the prenatal period leads to early onset of menarche and to delayed pubertal development [58].
The conclusion is that the onset of puberty occurs earlier in girls, and physiological variability and multiple other factors affect the onset of puberty. Exposure to a wide and growing range of known and unknown endocrine disruptors is ubiquitous, and changes in the onset of puberty may be influenced by exposures to endocrine disruptors at critical developmental windows. Endocrine disruptors are hormonally active substances that can act via several mechanisms to disrupt puberty either peripherally on the target organs (adipose tissue or adrenal glands) or centrally via the hypothalamic–pituitary–gonadal (HPG) axis. Nevertheless, the definitive evidence of associations between exposures to endocrine disruptors remains controversial [87, 88]. It seems obvious that some endocrine disruptors modify metabolic parameters: The increase in the latter [10] coincides with the increase in the prevalence of obesity with its risks over the last three decades and suggests that they are one of the major factors of the obesity epidemic [10]. The association between EDC and precocious puberty is subject to a bias that, as we have seen, is constituted by the improvement of health and nutritional conditions and the increase in the prevalence of obesity [89,90,91], which both can advance the age of puberty. However, current data are insufficient and conflicting to provide sufficient evidence for a causal relationship between exposure to endocrine disruptors and changes in the timing of puberty in humans. Definitive evidence for associations between exposures to endocrine disruptors remains controversial and still insufficient and contradictory to establish sufficient evidence for a causal relationship between exposure to endocrine disruptors and changes in the timing of puberty in humans. Further human epidemiological studies of a prospective and longitudinal nature are needed to determine the combined effect of EDC exposure on puberty and reproduction during critical periods. Furthermore, the underlying mechanisms by which early exposures to endocrine disruptors influence puberty, including epigenetic factors, need to be explored separately.
5.7 Endometriosis
Endometriosis is a common benign condition with potentially significant morbidity such as pelvic pain, dysmenorrhea, dyspareunia, and infertility and is thought to affect 2–50% of women of reproductive age [92, 93]. It is present in 71–87% of women with chronic pelvic pain [94].
The incidence and the prevalence associated with this disease showed an increasing trend in countries with a high sociodemographic index between 1990 and 2017 [92, 93]. Biologically, endometriosis is an estrogen-dependent, inflammatory, potentially chronic gynecological condition characterized by the proliferation of cells resembling functional endometrial tissue and growing outside the uterine cavity [95]. Despite the proposal of many theories, the precise etiology of the disease remains unknown. The oldest and still recognized hypothesis is the theory of retrograde menstruation [96]. Although the attachment of ectopic glands emanating from menstrual debris from reflux remains a plausible mechanistic explanation for the development of endometriosis, it does not explain all the incidences and presentation of the disease. Other theories regarding the development of endometriosis include coelomic metaplasia, activation of remnant stem cells, and inherent epigenetic abnormalities [97,98,99,100].
An additional difficulty is associated with the fact that endometriosis may take several different forms (ovarian endometrioma, peritoneal endometriosis, deeply infiltrating endometriosis, and adenomyosis—or endometriosis of the uterine muscle), which not only differ in location but also have different clinical presentations. In some cases, endometriosis remains asymptomatic, and a certain diagnosis can only be established by invasive evaluation (laparoscopy) and histopathological confirmation. Sometimes silent endometriosis is a condition in which the patient does not experience any discomfort resulting from the development of the disease, and symptoms may appear later in life or remain dormant.
Today, it appears that the development of endometriosis is determined by complex interactions between the composite effects of genetic and environmental risk factors. Indeed, families of genes associated with the immune system and inflammatory pathways, cell adhesion, and extracellular matrix remodeling have been described as being differentially expressed when comparing women with and without endometriosis [101, 102]. As a common environmental risk factor, endocrine-disrupting chemicals (EDCs) are ubiquitous in the environment and food chains and can affect the dynamic balance of sex hormones and mediate the innate dysregulation of immune cells, which may therefore play a role important in the pathogenesis of endometriosis [11, 103,104,105,106]. Nevertheless, there is a clear lack of well-established and modifiable risk factors for this disease; several existing publications have given conflicting results. There is therefore still no conclusive evidence for these potential risk factors regarding the combinations themselves or their management.
Because of the potential association between exposure to EDCs and the development of endometriosis, many studies have been devoted to this topic. Such studies are difficult to design, as it is difficult to identify both the study group and the control group and to measure the exposure to EDCs and the effects of other factors on the development of this condition.
Of the many EDCs, compounds that are best understood in terms of potential involvement in the pathogenesis of endometriosis are bisphenols [107], dioxin and dioxin-like compounds [25, 104], phthalates [108], and others.
5.8 Bisphenols
Bisphenol A (BPA) was the first to be synthesized, but evidences gathered in 1936 showed a low estrogen effect with affinity for the nuclear estrogen receptor. Its effects depend on dosage, targeted tissue, and tissue development on the site where it acts. The occurrence of estrogenic or antiestrogenic effects depends on the tissue targeted and on their impact on receptors [50]. Global production of BPA has steadily grown in the recent years on account of its multiple applications in the plastic and manufacturing industries, in food packaging, and in toys, causing a constant and permanent poisoning of food, water, and the environment. In 1950, it was found that bisphosphonates could be polymerized, and since then, they have been used to make polycarbonate plastics. These plastics have convenient features such as lightweight, moldability, and impact and heat resistance and are not susceptible to changes over time. About 20% of these plastics are used as a component of epoxy resin, serving as internal coating for plastic containers, bottles, and dental sealants. Therefore, it is a liquid and food contaminant present in abnormal levels in human serum analysis according to the literature. BPA is rapidly metabolized to inactive forms with a mean life cycle of approximately 4–5 h in adults, while in fetuses and children the metabolic rate is relatively low [109]. BPA can easily accumulate in adipose tissue for having lipophilic properties. Measurements of human serum have determined varied and controversial toxicity rates. Currently, the United States Environmental Protection Agency has established a safe level of 50 μg/kg/day, and the European Food Safety Authority has established a tolerable daily intake of less than 4 μg/kg/day. The list of products containing bisphenols available on the market has continued to grow, the most common being bisphenols BPS, BPF, BPB, and BPAF, which nevertheless seem to have the same properties.
Bisphenols are therefore estrogen-mimicking EDCs that are capable of maintaining low levels of progesterone receptors that can lead to disruptions in uterine cyclicity, a potential mechanism for the development of endometriosis [107]. The first, bisphenol A (BPA), previously used in the manufacturing of food cans and dental sealants, is one of the most well-studied and widespread EDCs.
Several previous experimental studies reported that the exposure of prenatal mice to bisphenol A (BPA) can cause endometriosis-like symptoms in offspring [110]. In human, it was abundantly present in sera of women with endometriosis compared with women without disease [111, 112]. A population-based case–control study to determine whether BPA exposure was linked to an increased risk of endometriosis, after measuring total urinary BPA concentrations in 143 cases (women with surgically diagnosed endometriosis) and 287 controls (women without a known endometriosis diagnosis), revealed a statistically significant, positive correlation between urinary BPA concentrations and peritoneal endometriosis, but not ovarian disease [113]. In contrast, in other studies, patients with ovarian endometriomas were found to have significantly higher urinary BPA concentrations than controls [112]. Other studies found no association between urinary [114, 115]. Inconsistencies among human studies likely reflect differences in populations, experimental design variations, and the rigorousness of the control groups [115].
5.9 Dioxins and Dioxin-Like Compounds
Dioxins and dioxin-like compounds are extremely resistant by-products of various industrial processes (e.g., waste incineration and iron/steel industries) or natural, and they represent ubiquitous environmental pollutants, chemically stable and lipophilic [116], and are polycyclic aromatic agents with chloral substituents.
Dioxins and dioxin-like compounds include the following:
-
(a)
Polychlorinated dibenzo-p-dioxins (PCDDs or dioxins): There are 75 PCDDs.
-
(b)
Seven of them are highly toxic polychlorinated dibenzofurans (PCDFs): There are 135 PCDFs. They are not dioxins, but ten of them have dioxin-like properties, the polychlorinated biphenyls (PCBs): There are 209 PCBs, and 12 of them have dioxin-like properties (the so-called coplanar PCBs because of the absence of chlorine substitution in ortho positions that gives the molecule a planar configuration). They have been widely used as dielectric and coolant fluids until they were banned worldwide in the 1980s [104].
PCDDs, PCDFs, and PCBs together form the group of polyhalogenated hydrocarbons and were found, by some authors, to be significantly associated with endometriosis [117, 118].
Dioxin generally enters the environment after accidents like the one in Seveso, Italy, in 1976. Dioxins then get into soil sediments, being carried by weather patterns, and become incorporated into the food chain [119]. They mainly enter the human body through food and, due to their lipophilic nature, accumulate in tissues with high-fat content [116]. Because of this property, it does not surprise to find high levels of dioxin and dioxin-like compounds in older people and reduced levels after delivery or breastfeeding [120]. Ten PCDFs, 12 PCBs (those with dioxin-like properties), and seven PCDDs bind to the aryl hydrocarbon receptor (AhR), an activated ligand transcription factor. AhR could be mostly found in the cytosol (sometimes in the nucleus) and represents the key component of the dioxin pathways [121]. In order to quantify their biological potency, all dioxin-like compounds have received a toxic equivalency factor (TEF) in terms of the most toxic dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]), which has a TEF of 1. However, the toxicity of a mixture of these compounds is often expressed in pg TEQ (toxic equivalent units)/g lipids, which represents the sum of the product of the concentration of each compound multiplied by its TEF [104]. The concentration is expressed per g lipids because they are mainly stored in adipose tissue [122].
The most toxic dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), due to its lipophilic nature, has the particularity of being very resistant to degradation and is able to modulate signaling processes mediated by estrogen and progesterone, steroid hormones necessary for the maintenance of normal uterine physiology. Exposure to TCDD has been experimentally linked to the development of reproductive disorders in mammals, most notably in a publication first reported by Rier in 1993, which found a positive correlation between exposure to TCDD and the incidence of endometriosis in a colony of rhesus monkeys [123]. Several studies have since been followed to examine the potential link between exposure to TCDD and the development of endometriosis [117, 124,125,126].
Concerning PCBs, within the reproductive tract, coplanar PCBs are particularly suited to act in concert with TCDD to disrupt key elements of communication between the immune and endocrine systems ([127, 128], potentially promoting reproductive disorders such as endometriosis. Rier, who had previously linked TCDD and endometriosis [123], subsequently reported a probable coexposure of these animals to significant levels of dioxin-like PCBs following food contaminated with toxic substances [129]. It therefore appears that, even within the framework of a controlled experimental study, it may be difficult to completely exclude additional occult sources of exposure to environmental toxicants via food or water [126, 129].
As with TCDD, although systematic review and meta-analysis results have shown that total PCBs are significantly associated with the risk of endometriosis, epidemiological data remain weak [130], or mixed [131], as for TCDD [126], with a number of studies failing to identify a clear association between TCDD exposure and endometriosis [115], even if certain authors concluded that a bad classification of the disease could have led to underestimating the risk [125].
5.10 Phthalates
Phthalates and their esters consist of a large group of chemical compounds with antiandrogenic and estrogenic activity frequently used in the plastic, coating, cosmetic, and toy industries and medical devices such as syringes and blood bags, and women are generally more at risk than men due to their employment in feminine care products and cosmetics [132]. Phthalates are the by-products of phthalic acid and are used in the plastics industry for their excellent moldability. In the roster of phthalates, three esters are considered endocrine disruptors with estrogenic effects: diethyl-hexyl phthalate (DHEP), benzyl-butyl phthalate (BBP), and dibutyl phthalate (DBP). Phthalates can be found not only in serum and human urine, but also in milk samples. Nevertheless, the mechanisms triggering the development of endometriosis by phthalates remain unclear. Tolerable daily intake ranges between 3 and 30 μg/kg/day [133,134,135]. In women with advanced endometriosis, significantly higher levels of mono-ethylhexyl phthalate (MEHP) and di-(2-ethylhexyl) phthalate (DEHP) were found in their plasma compared with disease-free women [136, 137]. The results of other studies, the National Health and Nutrition Examination Survey (NHANES), and the Endometriosis, Natural History, Diagnosis, and Outcomes study also revealed a significant association between urinary phthalates and endometriosis [115, 138]. Studies on the association between phthalate exposure and the presence of disease in Taiwanese women revealed a significant increase (p < 0.05) in urinary mono-n-butyl phthalate (MBP) and MEHP in patients with endometriosis [139, 140]. Nevertheless, other epidemiological studies failed to validate these findings. Upson [141], in a study including women from the northeast of the United States of America, showed an inverse association between the risk of developing endometriosis and levels of MEHP. These data were confirmed by Itoh [142] in a study of infertile women, although the authors only included 57 cases with endometriosis and 80 controls without endometriosis.
Despite suspicions of causation between phthalates and endometriosis, there are no regulations limiting their use in the United States or Brazil, although the European Community has banned them.
5.11 Medications as Endocrine Disruptors
5.11.1 Diethylstilbestrol
Historically, one of the most well-known pharmaceutical exposures to EDCs was the consequence of the consumption of diethylstilbestrol (DES) by pregnant women, which was originally prescribed with the aim of mitigating the risk of miscarriage, premature delivery, and other pregnancy-related complications [26]. DES is a synthetic, highly potent estrogen that was initially prescribed to women with high-risk pregnancies. Soon after, it was recommended to all pregnant women from the 1940s through the 1970s. In 1971, DES was banned in the United States because, in addition to being completely ineffective in preventing miscarriage, it was shown to increase the risk of serious illness in mothers and their children [143, 144].
Relevant to the current discussion, additional studies revealed an increased incidence of endometriosis in women whose mothers were prescribed DES compared with the daughters of women that were not given DES during pregnancy [145, 146].
5.12 Conclusion
The various studies concerning these three pathologies cited above, which show not only sometimes strong but also weak or contradictory relationships with endocrine disruptors, their involvement in complex metabolic disorders, and the new harmful effects on health of endocrine disruptors frequently used, highlight the full complexity of the problem. Taking this complexity into account in the assessment, management, and attempts to resolve it requires an approach from several points of view: environmental, ethical, scientific, epidemiological, economic, political, strategic, and preventive. Compounds potentially incriminated as endocrine disruptors are ubiquitous, present in our daily life (diet and lifestyle), increasing exponentially, persistent but also sporadic, and capable of producing potentially active metabolites. The scientific challenges are numerous due to the difficulties in dosing the compounds, the confusions, the complex mixtures of exposures and their interrelationships [147], the variability of the distributions of exposure from one study to another that can explain the differences in results, the design of numerous studies, and the imprecision of the exposure assessment methods (dosage, the number of patients, the duration of exposure, statistical bias, and difficulty in assaying the substances in question in the target organs), in particular for the chemicals with short half-life. In addition, biostatistical developments have not yet resulted in an ideal method to manage associated exposures that might exist in the human body [148]. Sometimes the limit values that can be considered toxic are unclear, and the relevance of animal models transferred to humans is questionable. Moreover, with the exception of evidence from accidentally exposed populations, experimental evidence demonstrates that developmental exposure to endocrine disruptors can lead to transgenerational adverse effects with health consequences: Such a concept is difficult to prove in humans because randomized designs of interventions to increase or decrease exposure are generally not applicable due to obvious ethical and logistical considerations.
A recurring theme in the studies reviewed is the appearance on the market of a colossal quantity of new substances, but also of their substitutes, little tested, wrongly assumed to be less toxic [15], and on the contrary revealing new signs of toxicity [26]. What about the recommended doses for BPA by the American Environmental Protection Agency for a safety level of 50 μg/kg/day, while the European Food Safety Authority has established a tolerable daily intake of less than 4 μg/kg/day? or concerning restrictions on phthalates, totally absent in the United States or Brazil, but banned by the European Community [149]? Are there divergences between financial interests and public health?
The otherwise justified terms “possible” or “probable” found in the literature for the risky should not obscure the precautionary principle, in light of reality: It is increasingly clear that endocrine disruptors are involved in diseases that are not transferable. Nevertheless, these synthetic compounds are ignored or at least underestimated as sustainable development goals (SDGs) of 2030, and decreasing exposure to synthetic chemicals with endocrine-disrupting or other harmful properties is not identified as one of the SDGs, although these rightly highlight that air pollution and climate change as global priorities [150] and despite the fact that intervention studies have produced rapid decreases in exposure to organophosphate pesticides, bisphenols, phthalates, parabens, and triclosans [151]. However, the decisions must come not only from the decision-makers, but also from the consumers. Since the majority of exposure to endocrine disruptors occurs through diet, choosing organic foods, lean meats, or a vegetarian lifestyle can help everyone minimize exposure. In addition, reducing the use of canned foods containing a BPA liner, using BPA-/BPS-free products, and avoiding long-term storage or heating of foods in plastic containers will also reduce the accidental exposure to the endocrine disruptors [26].
Therefore, in light of the above, clear-cut strategies and recommendations should be targeted to reduce human exposure to protect future generations from ever-increasing adverse health effects, and regulators should strengthen premarketing toxicological testing [152].
The need for additional further research is evident to further elaborate the effects of endocrine disruptors and other products on human health looking, of course, at causation and actions to reduce exposure to endocrine disruptors, taking into account the evidence and issues involved in decisions [153] and finding alternative manufacturing practices that can be applied to mitigate exposure to endocrine disruptors [24]. The additional costs to society can be weighed against the economic benefits of reduced disease and disability and other societal effects (e.g., ecosystem effects) [24], by always bearing in mind, however, that human health must take precedence over any other interest.
References
Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23.
Zoeller R, Brown TR, Doan LL, Gore AC, Shakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS, Endocrine-disrupting. Chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–110.
Pivonello C, Muscogiuri G, Nardone A, Garifalos F, Provvisiero DP, Verde N, De Angelis C, Conforti A, Piscopo M, Auriemma RS. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol. 2020;18:22.
Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33.
Wang Y, Zhu Q, Dang X, He Y, Li X, Sun Y. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecol Endocrinol. 2017;33:21–5.
Ding D, Xu L, Fang H, Hong H, Perkins R, Harris S, Bearden ED, Shi L, Tong W. The EDKB: an established knowledge base for endocrine disrupting chemicals. BMC Bioinformatics. 2010;11(Suppl 6):S5.
Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:e1–150.
Di Renzo GC, Conry JA, Blake J, et al. International federation of gynecology and obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynaecol Obstet. 2015;131:219–25.
WHO. International Programme on chemical safety. Global assessment of state-of-the-science for endocrine disruptors. Geneva: World Health Organization; 2012. https://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en. Accessed 6 Oct 2014.
Trasande L, Shaffer RM, Sathyanarayana S. Food additives and child health. Pediatrics. 2018;142:e20181408.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342.
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van der Saag PT, Van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63.
Simkova M, Vitku J, Kolatorova L, Jana Vrbkova J, Vosatkova M, Vcelak J, Dusskova M. Endocrine disruptors, obesity, and cytokines–how relevant are they to PCOS? Physiol Res. 2020;69(Suppl. 2):S279–93.
Žalmanová T, Hošková K, Nevoral J, Adámková K, Kott T, Šulc M, Kotíková Z, Prokešová Š, Jílek F, Králíčková M. Bisphenol S negatively affects the meotic maturation of pig oocytes. Petr J Sci Rep. 2017;7(1):485.
Eladak S, Grisin T, Moison D, Guerquin MJ, N'Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert RA. New chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103(1):11–21.
Błędzka D, Gromadzińska J, Wąsowicz W. Parabens. From environmental studies to human health. Environ Int. 2014;67:27–42.
Azzouz A, Colón LP, Hejji L, Ballesteros E. Determination of alkylphenols, phenylphenols, bisphenol A, parabens, organophosphorus pesticides and triclosan in different ceral based foodstuffs by gas chromatography-mass spectrometry. Anal Bioanal Chem. 2020;412(11):2621–31.
Kolatorova L, Duskova M, Vitku J, Starka L. Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol Res. 2017;66(Suppl 3):S305–15.
Kolatorova L, Vitku J, Hampl R, Adamcova K, Skodova T, Simkova PA, Starka LL, Duskova M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res. 2018;163:115–22.
Casey BJ, Tottennham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–10.
Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord. 2015;16(4):365–71.
Kawa IA, Masood A, Fatima Q, Mir SA, Jeelani H, Manzoor S, Rashid F. Endocrine disrupting chemical bisphenol A and its potential effects on female health. Diabetes Metab Syndr. 2021;15(3):803–11.
Papadimitriou A, Papadimitriou DT. Endocrine-disrupting chemicals and early puberty in girls. Children. 2021;8:492.
KahnL L, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8(8):703–18.
Polak G, Banaszewska B, Filip M, Radwan M, Wdowiak A. Environmental factors and endometriosis. Int J Environ Res Public Health. 2021;18:11025.
Rumph JT, Stephens VR, Archibong AE, Osteen KG, Bruner-Tran KL. Environmental endocrine disruptors and endometriosis. Adv Anat Embryol Cell Biol. 2020;232:57–78.
Stephens VR, Jelonia T, Rump JT, Amel S, Bruner-Tran KL. Osteen KG the potential relationship between environmental Endocrine disruptor exposure and the development of endometriosis and Adenomyosis. Front Physiol. 2022;12:1–15.
Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3.
Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8.
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9.
Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61.
Zawadzki J, Dunaif A. Current issues in endocrinology and metabolism: polycystic ovary syndrome. Cambridge MA: Blackwell Scientific Publications; 1992.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
Wang R, Mol BWJ. The Rotterdam criteria for polycystic ovary syndrome: evidence-based criteria? Hum Reprod. 2017;32:261–4.
Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R. Androgen excess: investigations and management. Best Pract Res Clin Obstet Gynaecol. 2016;37:98.
Livadas S, Pappas C, Karachalios A, Marinakis E, Tolia N, Drakou M, et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine. 2014;47:631–8.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97.
Diamanti-Kandarakis E, Livadas S, Katsikis I, Piperi C, Mantziou A, Papavassiliou AG, et al. Serum concentrations of carboxylated osteocalcin are increased and associated with several components of the polycystic ovarian syndrome. J Bone Miner Metab. 2011;29:201–6.
Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13–7.
Rachon D, Teede H. Ovarian function and obesity—interrelationship, impact on women's reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316:172–9.
Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–70.
Legro RS, Blanche P, Krauss RM, Lobo RA. Alterations in low-density lipoprotein and high-density lipoprotein subclasses among Hispanic women with polycystic ovary syndrome: influence of insulin and genetic factors. Fertil Steril. 1999;72:990–5.
Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95:2038–49.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800.
Cadagan D, Khan R, Amer S. Thecal cell sensitivity to luteinizing hormone and insulin in polycystic ovarian syndrome. Reprod Biol. 2016;16(1):53–60.
Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89(1):318–23.
Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–7.
Wijeyaratne CN, Seneviratne Rde A, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, Yapa C, Seneviratne Rde A, Balen AH. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist Endocrine Clinic. Hum Reprod. 2011;26(1):202–13.
Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med. 2018;11:191–207.
Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of Bisphenol a substitutes. Environ Health Perspect. 2015;123:643–50.
Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480–4.
Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51(2):165–9.
Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, Bellastella A, Carella C, Izzo A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101(6):1177–82.
Ebejer K, Calleja-Agius J. The role of cytokines in polycystic ovarian syndrome. Gynecol Endocrinol. 2013;29(6):536–40.
Mul D, De Muinck K-SSMPF, Oostdijk W, Drop SLS. Auxological and biochemical evaluation of pubertal suppression with the GnRH agonist leuprolide acetate in early and precocious puberty. Horm Res. 1999;51:270–6.
Cassio A, Cacciari E, Balsamo A, Bal M, Tassinari D. Randomised trial of LHRH analogue treatment on final height in girls with onset of puberty aged 7.5–8.5 years. Arch Dis Child. 1999;81:329–32.
Lebrethon MC, Bourguignon JP. Management of central isosexual precocity: diagnosis, treatment, outcome. Curr Opin Pediatr. 2000;12:394–9.
Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, Winneke G, Vanderschueren D, Staessen JA. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ Health Perspect. 2002;110:771–6.
Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4:254–64.
Parent AS, Franssen D, Fudvoye J, Gérard A, Bourguignon JP. Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: revision of human observations and mechanistic insight from rodents. Front Neuroendocrinol. 2015;38:12–36.
Zhu J, Kusa TO, Kusa TO, Chan YM. Genetics of pubertal timing. Curr Opin Pediatr. 2018;30:532–40.
Cheng G, Buyken AE, Shi L, Karaolis-Danckert N, Kroke A, Wudy SA, et al. Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev. 2012;70:133–52.
Krstevska-Konstantinova M, Charlier C, Crae M, Du Caju M, Heinrichs C, de Beaufort C, Plomteux G, Bourguignon JP. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod. 2001;16:1020–6.
Theodoropoulou S, Papadopoulou A, Karapanou O, Priftis K, Papaevangelou V, Papadimitriou A. Study of Xbal and Pvull polymorphisms of estrogen receptor alpha (ERα) gene in girls with precocious/early puberty. Endocrine. 2021;73(2):455–62.
Golestanzadeh M, Riahi R, Kelishadi R. Association of phthalate exposure with precocious and delayed pubertal timing in girls and boys: a systematic review and meta-analysis. Environ Sci Process Impacts. 2020;22:873–94.
Hashemipour M, Kelishadi R, Amin MM, Ebrahim K. Is there any association between phthalate exposure and precocious puberty in girls? Environ Sci Pollut Res Int. 2018;25:13589–96.
Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez M, Rybak M, Silva MJ, Ye X, Calafat AM, Kushi LH, et al. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod Toxicol. 2017;67:56–64.
Frederiksen H, Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, Skakkebaek NE, Andersson AM, Juul A. High urinary phthalate concentration associated with delayed pubarche in girls. Int J Androl. 2012;35:216–26.
Wolff MS, Teitelbaum SL, McGovern K, Windham GC, Pinney SM, Galvez M, Calafat AM, Kushi LH, Biro FM. Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum Reprod. 2014;29:1558–66.
Lomenick JP, Calafat AM, Melguizo Castro MS, Mier R, Stenger P, Foster MB, Wintergerst KA. Phthalate exposure and precocious puberty in females. J Pediatr. 2010;156:221–5.
Jung MK, Choi HS, Suh J, Kwon A, Chae HW, Lee WJ, Yoo EG, Kim HS. The analysis of endocrine disruptors in patients with central precocious puberty. BMC Pediatr. 2019;19:323.
Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, Vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4.
Rya BC, Hotchkiss AK, Crofton KM, Le G Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci Off J Soc Toxicol. 2010;114:133–48.
Durmaz E, Asci A, Erkekoglu P, Balcı A, Bircan I, Koçer-Gumusel B. Urinary bisphenol A levels in Turkish girls with premature thelarche. Hum Exp Toxicol. 2018;37:1007–16.
Supornsilchai V, Jantarat C, Nosoognoen W, Pornkunwilai S, Wacharasindhu S, Soder O. Increased levels of bisphenol A (BPA) in Thai girls with precocious puberty. J Pediatric Endocrinol Metab JPEM. 2016;29:1233–9.
Watkins DJ, Téllez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, Peterson KE, Meeker JD. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res. 2014;134:233–41.
Chen Y, Wang Y, Ding G, Tian Y, Zhou Z, Wang X, Shen L, Huang H. Association between bisphenol A exposure and idiopathic central precocious puberty (ICPP) among school-aged girls in Shanghai. China Environ Int. 2018;115:410–6.
Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, Peterson KE, Meeker JD. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res. 2017;159:143–51.
Leonardi A, Cofini M, Rigante DD, Lucchetti L, Cipolla C, Penta L. Esposito S the effect of bisphenol A on puberty: a critical review of the medical literature. Int J Environ Res Public Health. 2017;14:1044.
Vasiliu O, Muttineni J, Karmaus W. In utero exposure to organochlorines and age at menarche. Hum Reprod. 2004;19:1506–12.
Wohlfahrt-Veje C, Andersen HR, Schmidt IM, Aksglaede L, Sørensen K, Juul A, Jensen TK, Grandjean P, Skakkebaek NE, Main KM. Early breast development in girls after prenatal exposure to non-persistent pesticides. Int J Androl. 2012;35:273–82.
Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, De Caprio AP. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics. 2005;115:e127–34.
Windham GC, Pinney SM, Voss RW, Sjödin A, Biro FM, Greenspan LC, Stewart S, Hiatt RA, Kushi LH. Brominated flame retardants and other persistent organohalogenated compounds in relation to timing of puberty in a longitudinal study of girls. Environ Health Perspect. 2015;123:1046–52.
Harley KG, Rauch SA, Chevrier J, Kogut K, Parra KL, Trujillo C, Lustig RH, Greenspan LC, Sjödin A, Bradman A, et al. Association of prenatal and childhood PBDE exposure with timing of puberty in boys and girls. Environ Int. 2017;100:132–8.
Tassinari R, Mancini FR, Mantovani A, Busani L, Maranghi F. Pilot study on the dietary habits and lifestyles of girls with idiopathic precocious puberty from the city of Rome: potential impact of exposure to flame retardant polybrominated diphenyl ethers. J Pediatr Endocrinol Metab. 2015;28:1369–72.
Alyssa Huang A, Thomas Reinehr T, Christian L, Roth CL. Connections between obesity and puberty: invited by manuel tena-sempere, cordoba. Curr Opin Endocr Metab Res. 2020;14:160–8.
Lucaccioni L, Trevisani V, Marrozzini L, Bertoncelli N, Predieri B, Lugli L, Berardi A, Iughetti L. Endocrine-disrupting chemicals and their effects during female puberty: a review of current evidence. Int J Mol Sci. 2020;21:2078.
Papadimitriou A, Papadimitriou DT. Endocrine-disrupting chemicals and early puberty in girls. Children (Basel). 2021;8(6):492.
Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes. 2008;15:403–8.
Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–61.
Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019;3:44–54.
Kuznetsov L, Dworzynski K, Davies M, Overton C. Diagnosis and management of endometriosis: summary of NICE guidance. BMJ. 2017;358:j3935.
Moradi Y, Shams-Beyranvand M, Khateri S, Ghrahjeh S, Tehrani S, Varse F, Najmi Z. A systematic review on the prevalence of endometriosis in women. J Med Res. 2021;154(3):446–54.
Carpinello OJ, Sundheimer LW, Alford CE, Taylor RN, DeCherney AH. Endometriosis. In: Endotext. South Dartmouth (MA): MDText.com, Inc; 2017. 2000.
Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98(3):564–71.
Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–11043.
Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocr Rev. 2019;40:1048–79.
Baranova H, Canis M, Ivaschenko T, Albuisson E, Bothorishvilli R, Baranov V, et al. Possible involvement of arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1 genes in the development of endometriosis. Mol Hum Reprod. 1999;5:636–41.
Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:1.
Figueira PGM, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10.
Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88:1505–33.
Wren JD, Wu Y, Guo SW. A system-wide analysis of differentially expressed genes in ectopic and eutopic endometrium. Hum Reprod. 2007;22:2093–102.
Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F, Moscarini M. Impact of endocrine disruptor chemicals in gynaecology. Hum Reprod Update. 2008;14:59–72.
Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R. Environment and endometriosis: a toxic relationship. Eur Rev Med Pharmacol Sci. 2015;19:1964–72.
Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, et al. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: highlights from a National Italian Meeting. Int J Mol Sci. 2018;19:1647.
Sutton P, Woodruff TJ, Perron J, Stotland N, Conry JA, Miller MD, et al. Toxic environmental chemicals: the role of reproductive health professionals in preventing harmful exposures. Am J Obstet Gynecol. 2012;207:164–73.
Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96:175–9.
Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006;113:515–20.
Sartain CV, Hunt PA. An old culprit but a new story: bisphenol A and “NextGen” bisphenols. Fertil Steril. 2016;106:820–6.
Signorile PG, Spugnini EP, Citro G, Viceconte R, Vincenzi B, Baldi F, et al. Endocrine disruptors in utero cause ovarian damages linked to endometriosis. Front Biosci. 2012;4:1724–30.
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–12.
Rashidi BH, Amalou M, Lak TB, Ghazizadeh M, Eslami B. A case-control study of bisphenol A and endometrioma among subgroup of Iranian women. J Res Med Sci. 2017;22:7.
Upson K, Sathyanarayana S, De Roos AJ, Koch HM, Scholes D, Holt VL. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum Reprod. 2014;29:2457–64.
Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S. Urinary bisphenol-A concentration in infertile Japanese women and its association with endometriosis: a cross-sectional study. Environ Health Prev Med. 2007;12:258–64.
Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, et al. Bisphenol A and phthalates and endometriosis: the endometriosis: natural history, diagnosis and outcomes study. Fertil Steril. 2013;100:e1–2.
Van Den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansoson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41.
Heilier JF, Nackers F, Verougstaete V, Tonglet R, LiSon D, Donnez J. Increased dioxin-like compounds in the serum of women with peritoneal endometriosis and deep endometriotic (adenomyotic) nodules. Fertil Steril. 2005;84:305–12.
Porpora MG, Ingelido AM, Di Domenico A, Ferro A, Crobu M, Pallante D, Cardelli CEV, De Felipe E. Increased levels of polychlorobiphenyls in Italian women with endometriosis. Chemosphere. 2006;63:1361–7.
Kanematsu M, Shimuzu Y, Sato K, Kim S, Suzuki T, Park B, Hattori K, Nakamura M, Yabishita H, Yokota K. Distribution of dioxins in surface soils and river-mouth sediments and their relevance to watershed properties. Water Sci Technol. 2006;53:11–21.
Uemura H, Arisava K, Hikoshi M, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. PCDDs/PCDFs and dioxin-like PCBs: recent body burden levels and their determinants among general inhabitants in Japan. Chemosphere. 2008;73:30–7.
Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–8.
Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–6.
Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21:433–41.
Bois FY, Eskenazi B. Possible risk of endometriosis for Seveso, Italy, residents: an assessment of exposure to dioxin. Environ Health Perspect. 1994;102:476–7.
Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect. 2002;110:629–34.
Matta K, Lefebvre T, Vigneau E, Cariou V, Marchand P, Guitton Y. Associations between persistent organic pollutants and endometriosis: a multiblock approach integrating metabolic and cytokine profiling. Environ Int. 2021;158:106926.
Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters: what we have learned from Yusho disease. Environ Res. 2001;86(1):2–11.
Bruner-Tran KL, Osteen KG. Dioxin-like PCBs and Endometriosis. Syst Biol Reprod Med. 2010;56(2):132–46.
Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. Serum levels of TCDD and dioxin-like chemicals in Rhesus monkeys chronically exposed to dioxin: correlation of increased serum PCB levels with endometriosis. Toxicol Sci. 2001;59(1):147–59.
Zhang Y, Zheng X, Wang P, Zhang Q, Zhang Z. Occurrence and risks of PCDD/Fs and PCBs in three raptors from North China. Ecotoxicol Environ Saf. 2021;223:112541.
Cano-Sancho G, Ploteau P, Matta K, Adoamnei E, Buck Louis G, Mendiola J, Darai E, Squifflet J, Le Bizec B, Antignac JP. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: systematic review and meta-analysis. Environ Int. 2019;123:209–23.
Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–5.
Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 2015;6:8.
Fromme H, Gruber L, Seckin E, Raab U, Zimmermann S, Kiranoglu M, Schlummer M, Schwegler U, Smolic S, Völkel W, HBMnet. Phthalates and their metabolites in breast Milk results from the Bavarian monitoring of breast milk (BAMBI). Environ Int. 2011;37:715–22.
Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect. 2009;117:86–92.
Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: a prospective case control study. Fertil Steril. 2006;85:775–9.
Hartmann G. Are your personal care products disrupting hormonal balance? https://georgiahartmann.com/are-your-personal-care-products-disrupting-hormonal-balance. 2020.
Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999-2004. Environ Health Perspect. 2010;118:825–32.
Huang PC, Tsai EM, Li WF, Liao PC, Chung MC, Wang YH, et al. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod. 2010;25:986–94.
Huang PC, Li WF, Liao PC, Sun CW, Tsai EM, Wang SL. Risk for estrogen-dependent diseases in relation to phthalate exposure and polymorphisms of CYP17A1 and estrogen receptor genes. Environ Sci Pollut Res Int. 2014;21:13964–73.
Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL. Phthalates and risk of endometriosis. Environ Res. 2013;126:91–7.
Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S. Urinary phthalates monoesters and endometriosis in infertile Japanese women. Sci Total Environ. 2009;408:37–42.
Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99(2):134–46.
Harris RM, Waring RH. Diethylstilboestrol—a long term legacy. Maturitas. 2012;72(2):108–12.
Benagiano G, Brosens I. In utero exposure and endometriosis. J Matern Fetal Neonatal Med. 2014;27(3):303–8.
Upson K, Sathyanarayana S, Scholes D, Holt VL. Early-life factors and endometriosis risk. Fertil Steril. 2015;104(4):964–71.
Pollack AZ, Krall J, Kannan K, Buck Louis GM. Adipose to serum ratio and mixtures of persistent organic pollutants in relation to endometriosis: Findings from the ENDO Study. Environ Res. 2021;195:110732.
Bellavia A, James-Todd T, Williams PL. Approaches for incorporating environmental mixtures as mediators in mediation analysis. Environ Int. 2019;123:368–74.
Piazza MJ, Urbanetz AA. Environmental toxins and the impact of other endocrine disrupting chemicals in women's reproductive health. JBRA Assist Reprod. 2019;23(2):154–64.
Figueres C, Landrigan PJ, Fuller R. Tackling air pollution, climate change, and NCDs: time to pull together. Lancet. 2018;392:1502–3.
Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–20.
Kassotis CD, Vandenberg LN, Demeneix BA, et al. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020;8:719–30.
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Wenger, J.M., Marci, R. (2023). Endocrine Disruption in Women: A Cause of PCOS, Early Puberty, or Endometriosis. In: Marci, R. (eds) Environment Impact on Reproductive Health. Springer, Cham. https://doi.org/10.1007/978-3-031-36494-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-36494-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36493-8
Online ISBN: 978-3-031-36494-5
eBook Packages: MedicineMedicine (R0)