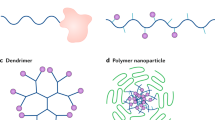
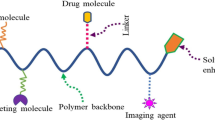
Abstract
Drugs, proteins, and nanoparticles delivered into the body are exposed to numerous mechanisms for premature elimination and deterioration within the body. Polymers can be covalently bound to this cargo as either bioactive or inert carriers of such therapeutics to impart protective characteristics. These include improved biodistribution, first-pass clearance reduction, specific targeting, and immune evasion. These polymers can be either synthetic or naturally derived and degradable or inert. Both the polymer and the cargo of interest may be joined together using a series of controlled and specific conjugation chemistries that target functional groups on either species to form covalent bonds. In addition, selectively cleavable linkers may be utilized to engineer cargo release at specific locations under unique physiological conditions such as low pH, oxygen, or biomolecule-laden environments. We will discuss not only different conjugation chemistry strategies and how they should be considered for targeted applications but also how one might reconcile them with different desired downstream characterization as well as in vivo uses.
Graphical Abstract

Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Su C, Liu Y, Li R et al (2019) Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv Drug Deliv Rev 143:97–114. https://doi.org/10.1016/j.addr.2019.06.008
Kostka L, Etrych T (2016) High-molecular-weight HPMA-based polymer drug carriers for delivery to tumor. Physiol Res 65:S179–S190. https://doi.org/10.33549/physiolres.933420
Melnyk T, Đorđević S, Conejos-Sánchez I, Vicent MJ (2020) Therapeutic potential of polypeptide-based conjugates: rational design and analytical tools that can boost clinical translation. Adv Drug Deliv Rev 160:136–169. https://doi.org/10.1016/j.addr.2020.10.007
Ko JH, Maynard HD, Angeles L, Angeles L (2019) Polymer Conjugates By Rational Design 47:8998–9014. https://doi.org/10.1039/c8cs00606g.A
Ekladious I, Colson YL, Grinstaff MW (2019) Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 18:273–294. https://doi.org/10.1038/s41573-018-0005-0
Longmire M, Choyke PL, Kobayashi H (2012) Clearance properties of Nano-sized particles and molecules as imaging agents. Consideration and Caveats. 3:703–717. https://doi.org/10.2217/17435889.3.5.703.Clearance
Larson N, Ghandehari H (2012) Polymeric conjugates for drug delivery. Chem Mater 24:840–853. https://doi.org/10.1021/cm2031569
Smilgies DM, Folta-Stogniew E (2015) Molecular weight-gyration radius relation of globular proteins: a comparison of light scattering, small-angle X-ray scattering and structure-based data. J Appl Crystallogr 48:1604–1606. https://doi.org/10.1107/S1600576715015551
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:1–19. https://doi.org/10.1186/2040-2384-2-14
D’souza AA, Shegokar R (2016) Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 13:1257–1275
Cruje C, Chithrani DB (2014) Polyethylene glycol functionalized nanoparticles for improved cancer treatment. Reviews in Nanoscience and Nanotechnology 3:20–30. https://doi.org/10.1166/rnn.2014.1042
Kopeček J, Yang J (2020) Polymer nanomedicines. Adv Drug Deliv Rev 156:40–64. https://doi.org/10.1016/j.addr.2020.07.020
Messina MS, Messina KMM, Bhattacharya A et al (2020) Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog Polym Sci 100:101186. https://doi.org/10.1016/j.progpolymsci.2019.101186
Winssinger N (2018) Bioorthogonal chemistry Chimia (Aarau) 72:A755. https://doi.org/10.1038/s43586-021-00028-z.Bioorthogonal
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Quiz Questions (Multiple Choice)
Quiz Questions (Multiple Choice)
-
Question 1: A positively charged hydrophilic PEG-small molecule dendrimer is found to have a hydrodynamic radius of 3 nm. Which of the following organs would play the biggest role in clearing the conjugate from a patient’s systemic circulation?
-
A.
Liver.
-
B.
Spleen.
-
C.
Kidney.
-
D.
Lungs.
-
A.
-
Answer: C. Kidneys have a size cutoff range of 6-8 nm. Factors such as charge can influence glomerular filtration as well.
-
Question 2: A protein-peptide conjugate is created to sterically block an immunogenic epitope on the protein. Thus, its diffusivity is similar to a protein of about 10−6 cm2/s. What would be the expected hydrodynamic radius of such a particle in water?
-
A.
2.27 x10−9 m.
-
B.
2.88 x10−8 m.
-
C.
2.13 x10−13 m.
-
D.
2.16 x10−6 m.
-
A.
-
Answer: A. First, the diffusivity of a protein is converted to m2/s. Using the Boltzmann constant of 1.38 x10−23 m2*kg*s−2*K−1, an average body temperature of 310 K, the viscosity of water (10−3 Pa) can all be plugged into the Stokes-Einstein equation. After converting the units, the denominator yields a value of 1.884x10−12 leaving all units cancelled while the numerator gives 4.278x10−21 m. Dividing these two values gives the final answer of 2.27x10−9 m or 2.27 nm radius.
-
Question 3: Your company is testing a new experimental branched pHPMA-drug conjugate that has hydrolytically degradable linkers along its backbone for controlled release of the drug. Which of the following purification modalities would likely NOT be suitable for your prototype therapeutic?
-
A.
Dialysis.
-
B.
Centrifugation.
-
C.
Size exclusion chromatography.
-
D.
Gel electrophoresis.
-
A.
-
Answer: A. Dialysis uses a semipermeable membrane to filter out unreacted components of a reaction using water. A hydrolytically degradable bond has the potential to be denatured or cleaved during this process.
Question 4: Your research lab hopes to create an amphiphilic micelle structure to increase the solubility of a hydrophobic drug. Which of the following assays would be most useful to characterize the shape of the conjugates. Additionally, which values indicate a spherical particle?
Dynamic Light scattering, Rg/RH > 0.775.
-
A.
Small angle x-ray diffraction and dynamic light scattering, Rg/RH < 0.775.
-
B.
Small angle x-ray diffraction, Rg/RH > 0.775.
-
C.
Small angle x-ray diffraction and dynamic light scattering, Rg/RH > 0.775.
-
A.
-
Answer: B. DLS gives hydrodynamic radius RH while small angle x-ray diffraction gives Rg. Thus, both would be needed. The ratio of Rg/RH for a globular (circular) particle is less than 0.775.
-
Question 5: A 100 Da PEG chain has a monomer length of 3.5 Å. If a nanoparticle has a radius of 10 nm. The distance between PEG monomers is 100 Å. which of following confirmations would the pegylated nanoparticle take?
-
A.
Mushroom.
-
B.
Brush.
-
C.
The particle will have both mushroom and brush confirmations.
-
D.
Not enough information.
-
A.
-
Answer: A. Using the equation for the Flory Radius yields 55.47 Å. Since the RF is lower than 100 Å, the confirmation of the coating will likely be mushroom.
-
Question 6: You are designing a drug that disrupts the metabolic pathway of a cancer cell and have decided to use a degradable linker to facilitate intracellular release. Which of the following is the least suitable for this task?
-
A.
Hydrazide (pH).
-
B.
Oligo-peptide chain (enzyme).
-
C.
Ester bonds (hydrolytic).
-
D.
Disulfide bond (reduction).
-
A.
-
Answer: C. Engineered intracellular release can be facilitated by using bonds sensitive to cleavage of enzymes, lower pH, and a reducing environment. Hydrolysis is a less ideal method due to potential degradation in systemic circulation.
-
Question 7: A dendrimer contains encapsulated Doxorubicin (DOX) and you’re tasked with studying the release of the drug over time. Doxorubicin is fluorescent when it leaves the nanoparticle. The polymer has the composition such that it is comprised of hydrolytically cleavable ester bonds. You want to prevent burst release of DOX from these nanoparticles. Which of the following rates would best represent such as release?
-
A.
τreaction > τdiffusion.
-
B.
τreaction < τdiffusion.
-
C.
τreaction = τdiffusion.
-
D.
there is no difference in outcomes.
-
A.
-
Answer: B. Burst release can be characterized by a rapid cleavage of the hydrolytically degradable bond such that the rate (τreaction) is larger than diffusion (τdiffusion). Thus, for slower release of the drug, τreaction < τdiffusion.
-
Question 8: You’re studying different molecular weight PEG chains to increase the hydrodynamic radius of a chemotherapeutic. However, with increased retention time, your tasked to test possible toxicity relating to these new formulations. Which of the following assays would NOT be suitable?
-
A.
Live/dead viability in vitro.
-
B.
MTT assay.
-
C.
ATS/ALS ELISA.
-
D.
Live fluorescent imaging.
-
A.
-
Answer: D. Live fluorescent imaging is useful for tracking a particle in vivo in real time. It would not be ideal to investigate toxicity.
-
Question 9: Which of the following are NOT considerations when conjugating proteins?
-
A.
Reduction of functionality due to steric hindrance.
-
B.
Similar molecular weight of polymer chains leading to difficulty characterizing.
-
C.
Linkers must be used to bind the initiator to the functional group.
-
D.
Neighboring amino acid residues may lead to off targeting chemistries.
-
A.
-
Answer: C. Linkers do not necessarily have to be used in protein conjugation strategies. Polymers may be covalently bound to proteins using a functional amino acid residue and a functional group on the polymer and or initiator.
-
Question 10: You are researching possible amino acid residues to target on your protein for conjugation to a biomaterial carrier. You determine that you need a site-specific reaction for this. Which of the following would NOT be appropriate?
-
A.
Reductive amination of a lysine group.
-
B.
Disulfide exchange of a cysteine.
-
C.
Recombination of the protein to introduce a non-canonical amino acid.
-
D.
Enzymatic ligation of a C-terminus.
-
A.
-
Answer: A. Lysine is generally the most abundant amino acid on the surfaces of proteins. Thus, conjugation strategies targeting lysines are usually performed for the purpose of speckled coatings and not for epitope or site-specific reactions.
Rights and permissions
Copyright information
© 2023 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Quiroz, V.M., Devier, J., Doloff, J.C. (2023). Therapeutic Polymer Conjugates and Their Characterization. In: Domb, A., Mizrahi, B., Farah, S. (eds) Biomaterials and Biopolymers . AAPS Introductions in the Pharmaceutical Sciences, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-031-36135-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-36135-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36134-0
Online ISBN: 978-3-031-36135-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)