Abstract
It all began in Weimar, at the Goethe National Museum in Weimar, a magnificent and placid place, redolent in history and culture, where Prof. Richard Baum organized the fourth Mitteldeutsches Neuroendokriner Tumor Symposium, in June 2013 (Fig. 7.1). Richard has always been a pioneer and the first to understand and disseminate the importance of many innovations, including the now popular theranostic concept. Unlike many conservative and “predictable” scientific gatherings, his meetings have always been avant-garde and pivotal in defining the trends for the future. To my delight and pleasure, I was invited to participate in what I knew would be a tour de force of the trailblazers of innovative nuclear medicine. Inspired by such greatness, we subsequently established the LuGenIum Consortium for Independent Research to addressed some of the major challenges in the use of peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors (NETs), which are the prediction of efficacy and toxicity and the consequent patient stratification. In the following years, we conducted clinical trials to understand the role of individual predisposition and specific tumor genomic profile in the response and toxicity to PRRT. Our specific aims were to, first, conduct a retrospective analysis of toxicity to PRRT (i.e., define the problem) in a large patient cohort, then to conduct two prospective studies (assess the efficacy of the ideas). To this aim, we firstly identified NETs at a genomic level and assessed the response to PRRT with a PCR-based blood analysis, and, secondly, we analyzed markers of long-term toxicity in patients previously undergone to PRRT, with a PCR-based blood analysis. During our investigations, we identified a new biomarker, the PRRT predictive quotient, or PPQ, which demonstrated 95% accuracy in predicting the response to PRRT in the three prospective series that were studied under the LuGenIum umbrella. These results provide the demonstration that PPQ is a predictive biomarker and a measure of radiosensitivity. We also demonstrated in these patients that the NET identifying circulating transcriptomic signature NETest was able to accurately monitor the course of PRRT during its delivery, as opposed to imaging, which becomes reliable only after treatment completion. The results of these studies opened a new era for treatment individualization and optimization.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
It all began in Weimar, at the Goethe National Museum in Weimar, a magnificent and placid place. The place is redolent in history and culture, where Prof. Richard Baum organized the fourth Mitteldeutsches Neuroendokriner Tumor Symposium, in June 2013 (Fig. 7.1). Richard has always been a pioneer and the first to understand and disseminate the importance of many innovations, including the now popular theranostic concept. Unlike many conservative and “predictable” scientific gatherings, his meetings have always been avant-garde and pivotal in defining the trends for the future. To my delight and pleasure, I was invited to participate in what I knew would be a tour de force of the trailblazers of innovative nuclear medicine.
After more than 13 years of full immersion in peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors (NETs), I was in search of new inspirations to improve this excellent treatment. I had been captured by the possibilities of accurate monitoring and prediction of therapy provided by blood transcriptome signatures for NETs, as proposed by the inventor of this field, Prof. Irvin M. Modlin of Yale University [1]. While walking through the rooms of Goethe’s house, and inspired by the book and art collection that is so well-preserved there, I initiated a peripatetic discussion about the future of PRRT with Richard, Irvin, and Dik (the late and much beloved Prof. Dik J. Kwekkeboom from Erasmus University), who established 177Lu-DOTATATE therapy. In the green study of the Master, I realized that three of the most important minds in the field of neuroendocrinology were there with me. “What if we could apply the sophisticated genomic techniques to PRRT?” I asked. The idea was met with considerable interest as well as skepticism. We then decided to meet in Lyon, during the EANM ’13 annual meeting. There, among pâté de foie gras and a glass of Côtes de Gascogne, we decided to establish and fund a research group. In honor of the city of Lyon, whose old name was Lugdunum, we established LuGenIum (Fig. 7.2).
The scope of our innovative research venture was to understand the role of individual predisposition and specific tumor genomic profile in the response and toxicity to PRRT. Our specific aims were to, first, conduct a retrospective analysis of toxicity to PRRT (i.e., define the problem) in a large patient cohort, then to conduct two prospective studies (assess the efficacy of the ideas). Firstly, to identify NETs at a genomic level and assess the response to PRRT with a PCR-based blood analysis, and, secondly, to analyze markers of long-term toxicity in patients with NETs previously undergone to PRRT with a PCR-based blood analysis.
7.1 Retrospective Analysis of Toxicity
Given the increased use of PRRT in NETs and the numerous other therapies in patients undergoing PRRT, it is crucial to define the risk relationship between toxicity and therapy by identifying the risk factors. These factors are considered, by the clinicians, responsible of modulating the occurrence of adverse events after PRRT by altering the thresholds for toxicity to critical organs.
We assessed 807 subjects enrolled at my institution (at that time), the European Institute of Oncology (IEO) in Milan, who had received PRRT with 90Y-, 177Lu-, and 90Y+177Lu-somatostatin analog peptides. Our concept was to evaluate the renal and bone marrow toxicity, expressed by blood chemistry analysis, and the parameters then considered to modulate the tolerability, such as the risk factors, PRRT parameters, and clinical features [2]. To ensure a balanced assessment we utilized sophisticated statistical analysis with multiple regression, random forest feature selection, and recursive partitioning and regression trees.
We observed that severe nephrotoxicity was virtually absent after 177Lu-peptides and was related to the administration of 90Y-peptides. G1/G2 creatinine toxicity was present in 34.6% of all patients and G3/G4 toxicity in 1%. None of the patients treated with only 177Lu-DOTATATE, however, developed severe toxicity. Bone marrow toxicity was low and comparable with other anti-neoplastic therapies. Myelodysplastic syndrome was observed in 2.35% of individuals, with a minority developing acute leukemias (1.1%). More interestingly, in our comparative analysis of nephrotoxicity, it was apparent that clinical factors, such as hypertension or prior nephrotoxic chemotherapy, as well as clinical features, such as anemia, failed to provide a basis for more than 34% of the cases of toxicity. Similarly, in our comparative analysis of hematotoxicity, clinical factors, such as prior myelotoxic chemotherapies or bone marrow invasion, as well as clinical features, such as thrombocytopenia, could only be incriminated in ~30% of the cases of myeloproliferative disease. These data strongly suggest the existence of unidentified individual susceptibilities to radiation-associated disease, most likely of a genetic basis. Our inescapable conclusion was that personalized molecular approaches would be required to identify individual radiosensitivity.
7.2 Circulating NET Transcripts
The NETest is a gene expression assay that measures 51 NET marker transcripts in blood using real-time PCR [1, 3]. The 51 NETest genes are included in “14 omes”. The assay utilizes multi-algorithmic analysis to quantify expression of gene clusters related to the tumor. NETest output is a score scaled 0–100 that represents the risk of NET disease. A normal score is ≤20, stable disease 21–40, progressive disease 41–100. The assay has demonstrated >90% accuracy, sensitivity and specificity for a NET diagnosis and residual/recurrent disease in numerous prospective studies and a recent meta-analysis [4,5,6,7,8,9].
7.3 Circulating NET Transcripts and SSR Imaging
The concept of adding an mRNA-based omic strategy to PRRT was based on our recognition of the need to improve the diagnostic and therapeutic approach to NETs. There was an obvious requirement to move from a mono-dimensional approach based on a single piece of information (i.e., somatostatin receptor expression) to a multidimensional one, based on the multiple simultaneous molecular measurements of the genes regulating tumor biology (behavior). We felt this was accomplishable if we could use the 51 “NET-defining” gene transcripts and their omic clusters to genomically characterize individual tumors.
Initially, we evaluated the relationship of the NETest with diagnostic imaging, namely Ga-68-based somatostatin analog PET (68Ga-SSA-PET) [10]. Our hypothesis was that the integration of circulating molecular markers and a tissue index of proliferation with functional imagery would provide added functional information in respect of tumor biology and clinical behavior. We recruited two independent patient groups affected by gastroenteropancreatic (GEP) and bronch-pulmonary (BP) NETs with positive 68Ga-SSA-PET and evaluated all with NETest: 27 patients pre-PRRT as primary or salvage treatment from two Italian institutions, IEO, Milan and Istituto Tumori della Romagna (IRST), Meldola, and 22 patients referred for staging/restaging after various therapies at Charité University, Berlin. To understand the relationship between gene expression and imaging, we examined the maximum standardized uptake value (SUVmax) at PET and the circulating gene transcripts. Additional parameters included Ki-67 index, Chromogranin-A (CgA). Transcripts were measured by real-time quantitative reverse transcription PCR (qRT-PCR) and multianalyte algorithmic analysis, CgA by enzyme-linked immunosorbent assay (ELISA). Statistical analysis to evaluate the strength of the relationships with the NETest included regression analyses, generalized linear modeling, and receiver-operating characteristic (ROC) curves.
Firstly, our regression model confirmed that the SUVmax measured in two centers were comparable. NETest was positive in 47 of 49 patients (96%), CgA was positive in 26 (54%) (χ2 = 20.1, p < 2.5 × 10−6, and 78% were G1-G2 according to WHO 2010 (Ki-67 < 20%). Gene transcript scores were predictive of imaging with >95% concordance and significantly correlated with SUVmax (R2 = 0.31, root-mean-square error = 9.38). This meant that specific genes accurately predict the uptake: the genes MORF4L2 and HSF2, followed by somatostatin receptors SSTR1, 3, and 5 exhibited the highest correlation with SUVmax. Progressive disease was identified by elevated levels of a quotient of MORF4L2 expression and SUVmax [ROC-derived AUC (R2 = 0.7, p < 0.05). As expected, no statistical relationship was identified between CgA and Ki-67 and imaging parameters (Fig. 7.3).
Neuroendocrine specific multitranscriptomic analysis, NETest, predicts correlates with and predicts uptake at 68Ga-labeled somatostatin analogue (SSA) PET with great concordance ((a) >95% concordance, R2 = 0.31, root-mean-square error = 9.38). (b) Typical appearance of a 68Ga-DOTATOC PET scan, maximum intensity projection (MIP) in a patient with elevated NETest, demonstrating an intensely avid pancreatic lesion (dotted arrow) as well as in intensely avid liver metastasis (solid arrow). (c) Histogram illustrating the genes contributing to predict the uptake at 68Ga-SSA-PET: MORF4L2 and HSF2, are the most important. (d) Progressive disease was identified by elevated levels of a quotient of MORF4L2 expression and SUVmax [ROC-derived AUC (R2 = 0.7, p < 0.05). A circulating gene-based quotient, therefore, has relevance for clinical management, because it adds functional biological multi-dimensionality to an image
7.4 Circulating NET Transcripts and PRRT
We then moved on and tested the correlation of NETest and PRRT efficacy. Specifically, the aim of this segment of the research was to assess the accuracy of circulating NET transcripts as a measure of PRRT efficacy, and to identify prognostic gene clusters in baseline blood that could have relevance for PRRT efficacy [11]. Our hypothesis was that the measurement of circulating NET transcripts of patients undergoing PRRT would enable assessment of tumor response and provide biologically relevant information on an individual tumor. We prospectively enrolled 54 subjects with GEP and BP NETs. The majority (47/54) had low-grade NETs (G1/G2; BP typical/atypical), 31/49 were 18FDG positive and 39/54 had progression at start. Disease status was assessed by RECIST1.1. Statistical analysis included chi-square, non-parametric measurements, multiple regression, receiver operating characteristic, and Kaplan-Meier survival curves. The disease control rate (stability, partial and complete responses) was 72% and median progression-free survival (PFS) was not reached (median follow-up: 16 months). Only grading (but not CgA, SSR expression, or FDG positivity) was associated with response (p < 0.01). At baseline, 94% of patients were NETest-positive, while CgA was elevated in 59%. NETest accurately (89%, χ2 = 27.4; p = 1.2 × 10−7) correlated with treatment response, while CgA was only 24% accurate. Additionally, we observed that pre-treatment expression of 8 genes representing clusters of genes regulating two components of tumor biology, namely growth-factor signaling (GFS) and metabolism (MTb), correlated with response. GFS and MTb omic clusters exhibited an AUC of 0.74 ± 0.08 (z-statistic = 2.92, p < 0.004) for response prediction (76% accuracy). Ki67 alone had no value as a predictor of treatment efficacy. To amplify the clinical utility using all modalities, we used a logistic regression model to integrate the GFS/MTb parameter with grading. This provided a binary treatment prediction output: “predicted responder” (PPQ+); “predicted non-responder” (PPQ−) with an AUC of 0.90 ± 0.07, irrespective of tumor origin. The newly defined PRRT predicting quotient (PPQ) exhibited a 94% accurate correlation with PRRT responders (SD + PR + CR; 97%) vs. non-responders (91%).
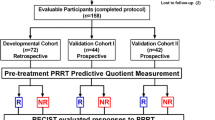
7.5 Validation Study of PRRT Genomic Signature in Blood (PPQ) for the Prediction of 177Lu-octreotate Efficacy
The characterization of the PPQ in the discovery cohort was the first demonstration of a pre-treatment parameter able to correlate with high accuracy with the response to PRRT. In so doing it accomplished longstanding unmet need in the radionuclide treatment of NETs, namely on a biological basis to accurately predict therapeutic efficacy. This parameter however needed validation in larger, independent cohorts to demonstrate that PPQ (an algorithm that integrates circulating NET-specific transcripts with tissue Ki67), would be able to differentiate PRRT-responders from PRRT-non-responders prior to the initiation of PRRT. In this respect, it was important to demonstrate that PPQ would behave as a predictive and not as a prognostic biomarker, by confirming that PPQ correlated only with PRRT response and not to other treatment strategies.
The study of PPQ as a predictive biomarker was undertaken in three independent 177Lu-PRRT treated cohorts with a total of 158 subjects: the original developmental cohort, now enlarged to 72 subjects from IRST Meldola, Italy, and the two prospective validation cohorts from Zentralklinik Bad Berka, Germany (n = 44), and Erasmus Medical Center, Rotterdam, Netherlands (n = 42). Each cohort included predominantly well-differentiated, low-grade (G1–G2, 86–95%) GEP and BP NETs. To demonstrate the specificity of PPQ, we included two non-PRRT comparator cohorts: SSA-treated cohort I (n = 28; 100% G1–G2, 100% GEP) and II (n = 51; 98% low grade; 76% GEP-NET), and a watchful-waiting cohort (n = 44; 64% G1–G2; 91% GEP). Baseline parameters to be tested included PPQ, disease status, SSR, and CgA. Treatment response was evaluated using RECIST criteria [responder (stable, partial, and complete response) vs non-responder)]. Sample measurement and analyses were blinded to study outcomes. Statistical evaluation included Kaplan-Meier survival and standard test evaluation analyses.
In the developmental cohort, 56% responded to PRRT. The PPQ predicted 100% of responders and 84% of non-responders, with an accuracy of 93%. The two validation cohorts exhibited a response of 64 and 79%, respectively. In both, the PPQ was 95% accurate (Bad Berka: PPQ+ = 97%, PPQ− = 93%; Rotterdam: PPQ+ = 94%, PPQ− = 100%). Overall, the median PFS was not reached in PPQ+ vs PPQ− (10–14 months; HR: 18–77, p < 0.0001). In the two comparator cohorts, where SSA-treatment (n = 79) and watchful waiting (n = 44) were applied, the PRRT predictor (PPQ) had an accuracy of 47 and 50%, respectively. Essentially, the predictive accuracy of flipping a coin! In addition, the PFS of the PPQ+ and PPQ− did not exhibit any significant differences in any of the two comparator cohorts. These data demonstrated that the PPQ measurement is an accurate predictor of radiosensitivity (Fig. 7.4).
PRRT Prediction Quotient for PFS prediction in PRRT-treated and non-treated cohorts. In the PRRT-treated cohorts (a–c) positive PPQ predicted a favorable PRRT outcome and was associated with undefined mPFS, while negative PPQ predicted a poor PRRT outcome and was associated with a mPFS of 11–14 months. In the comparator, non-PRRT treated cohorts (d–f), the prediction of PPQ did not make any difference in the outcome of therapy, resulting in similar mPFS for the PPQ positive and negative subjects. These results provide the demonstration that PPQ is a predictive biomarker and a measure of radiosensitivity
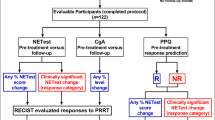
7.6 Validation Study of Multigene NET-Specific Circulating Transcript Signature for the Monitoring of 177Lu-octreotate Efficacy
Finally, we sought to validate the correlation of the multigene NET-specific circulating transcript signature with efficacy. Specifically, it was our intention to test the hypothesis that the NETest is able to over time monitor the response to PRRT and provide added value to the PPQ [12]. We prospectively evaluated whether the NETest was a surrogate biomarker for RECIST in defining disease response and if NETest levels correlated with pretreatment PPQ prediction of efficacy. We included 122 prospectively enrolled patients with GEP and BP NETs undergoing PRRT with 177Lu-peptides. These were divided into three cohorts (IRST, Meldola, Italy: n = 72; Zentralklinik Bad-Berka, Germany: n = 44; Erasmus University Rotterdam, Netherlands: n = 41). NETest was measured at baseline, at each PRRT cycle, and at follow-up (2–12 months). NETest is defined by a 1–100 score: stable disease is identified by a score <40, progression >40. CgA was used as a comparator. Samples were de-identified, and measurement and analyses were blinded. Kaplan-Meier survival and standard statistics were assessed. RECIST stabilization or response (“responder”) occurred in 67%; 33% progressed. NETest significantly (p < 0.0001) decreased in “responders” (−47 ± 3%); in “non-responders,” NETst levels remained elevated (+79 ± 19%) (p < 0.0005). NETest monitoring accuracy was 98% (119/122). NETest levels >40 in the follow-up (indicating a progressive status) correlated with a shorter mPFS (10 months), as opposed to stable NETest levels (<40; not reached; HR 0.04 (95%CI, 0.02–0.07). PPQ was confirmed to predict response with high accuracy (118/122, 97%) with a 99% accurate positive and 93% accurate negative prediction. The combination of information deriving from the two genomic biomarkers demonstrated that NETest significantly (p < 0.0001) decreased in PPQ-predicted responders (−46 ± 3%). Conversely, in PPQ-predicted non-responders the NETest remained elevated or increased (+75 ± 19%). Follow-up NETest values, stable vs progressive, reflected the PPQ prediction and the mPFS (not reached vs. 10 months; HR 0.06 (95%CI, 0.03–0.12). CgA was noncontributory: it decreased in 38% of PRRT responders and 56% of non-responders (p = NS). In summary, these studies demonstrated two major outcomes. Firstly, the PPQ predicted PRRT response in 97%; secondly, the NETest accurately monitored PRRT response. Overall, it was evident that the PPQ is an effective predictive biomarker specific for PRRT and that the NETest provides an effective real-time surrogate marker of PRRT radiological response (Fig. 7.5). This is particularly valuable in light of the frequent pseudo-progression, related to the radiation inflammatory response, which limits the evaluation of PRRT outcome until after the end of PRRT [13]. It also provides evidence for further consideration in respect of health economic impact of repeated imaging as opposed to the use of a non-invasive biomarker assessment [14].
NETest levels during PRRT. PRRT responders (blue, a, b) had significantly decreased NETest levels during and after PRRT. A decrease to NETest stable levels (NETest < 40) was significantly associated with a favorable outcome, represented by an undefined mPFS (blue, c). On the contrary, patients who did not respond to therapy (red, a, b) exhibited significantly increased NETest levels during and after PRRT. Increasing NETest levels to the progressive range (>40) were significantly associated with a poor outcome of PRRT, represented by a mPFS of 10 months (red, c). This is particularly valuable in light of the frequent pseudo-progression, related to the radiation inflammatory response, which limits the evaluation of PRRT outcome until after the end of PRRT
7.7 Future Developments
PRRT has demonstrated efficacy and tolerability in the treatment of well-differentiated neuroendocrine tumors. The work of the LuGenIum Consortium for Independent Research addressed some of the major challenges in its use, which are the prediction of efficacy and toxicity and the consequent patient stratification. Transcriptomic evaluations of blood and a combination of gene expression and specific SNPs, aided by machine learning algorithms, are worth consideration as key strategies to provide molecular tools that will enhance the efficacy and safety of PRRT [15].
References
Modlin IM, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8:e63364.
Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19.
Modlin IM, Drozdov I, Alaimo D, et al. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr Relat Cancer. 2014;21:615–28.
Oberg K, Modlin IM, De Herder W, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:435–46.
Pavel M, Jann H, Prasad V, Drozdov I, Modlin IM, Kidd M. NET blood transcript analysis defines the crossing of the clinical rubicon: when stable disease becomes progressive. Neuroendocrinology. 2017;104:170–82.
Modlin IM, Aslanian H, Bodei L, Drozdov I, Kidd M. A PCR blood test outperforms chromogranin A in carcinoid detection and is unaffected by proton pump inhibitors. Endocr Connect. 2014;3:215–23.
Pacak K, Kidd M, Meuter L, Modlin IM. A novel liquid biopsy (NETest) identifies paragangliomas and pheochromocytomas with high accuracy. Endocr Relat Cancer. 2021;28(11):731–44.
Modlin IM, Kidd M, Falconi M, et al. A multigenomic liquid biopsy biomarker for neuroendocrine tumor disease outperforms CgA and has surgical and clinical utility. Ann Oncol. 2021;32(11):1425–33.
Modlin IM, Kidd M, Frilling A, et al. Molecular genomic assessment using a blood-based MRNA signature (NETest) is cost-effective and predicts neuroendocrine tumor recurrence with 94% accuracy. Ann Surg. 2021;274:481–90.
Bodei L, Kidd M, Modlin IM, et al. Gene transcript analysis blood values correlate with 68Ga-DOTA-somatostatin analog (SSA) PET/CT imaging in neuroendocrine tumors and can define disease status. Eur J Nucl Med Mol Imaging. 2015;42:1341–52.
Bodei L, Kidd M, Modlin IM, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2016;43:839–51.
Bodei L, Kidd MS, Singh A, et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging. 2020;47:895–906.
Brabander T, van der Zwan WA, Teunissen JJM, et al. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [(177)Lu-DOTA(0),Tyr(3)]octreotate. Endocr Relat Cancer. 2017;24:243–51.
Liu E, Paulson S, Gulati A, et al. Assessment of NETest clinical utility in a U.S. Registry-based study. Oncologist. 2019;24:783–90.
Bodei L, Schöder H, Baum RP, et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020;21:e431–43.
Acknowledgments
These results have been made possible by the support, knowledge, inspiration, and vision of Dik J. Kwekkeboom (of beloved memory), Eric P. Krenning, Irvin M. Modlin, and Richard P. Baum. I am also profoundly indebted to Irvin Modlin, Mark Kidd, and Ignat Drozdov who developed the NETest and whose felicitous intellectual support facilitated my work in the development of the PPQ. My gratitude also goes to my collaborators from Italy, particularly Giovanni Paganelli, Chiara M. Grana, and Stefano Severi; from Bad Berka, particularly Aviral Singh; and from Rotterdam, particularly, Wouter van de Zwan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Bodei, L. (2024). The LuGenIum Triptych: Ode to a Theranostic Transcriptome. In: Prasad, V. (eds) Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum. Springer, Cham. https://doi.org/10.1007/978-3-031-33533-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-33533-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33532-7
Online ISBN: 978-3-031-33533-4
eBook Packages: MedicineMedicine (R0)