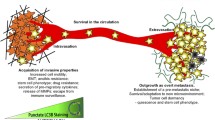
Abstract
During cancer development, tumour cells are exposed to various intrinsic and extrinsic stresses such as nutrient deficiency, lack of oxygen, DNA damage, and growth factor deprivation that regulate cell growth and homeostasis. In response to these stresses, tumour cells, unlike healthy cells, develop adaptive strategies to grow and migrate successfully. One of the key mechanisms that cancer cells utilize to circumvent cellular stresses is autophagy, which is a catabolic process that facilitates the degradation and recycling of damaged organelles, thereby reducing cellular stress and promoting cell survival. Emerging studies have shown a vital role of autophagy in cancer metastasis, which is the major cause of cancer-associated deaths. However, the role of autophagy in metastasis is multidimensional and involves both metastasis-promoting and suppressing roles dependent on the demands of tumour cells during the metastatic process. As novel compounds targeting autophagy emerge, it will be crucial to consider the stage of metastatic progression at which autophagy is being targeted to efficiently overcome metastasis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- APP:
-
Amyloid precursor protein
- ARHI:
-
Aplasia Ras homolog member I
- ATF:
-
Activating transcription factor
- ATG:
-
Autophagy-related gene
- CAF:
-
Cancer-associated fibroblasts
- CDCP1:
-
CUB domain-containing protein-1
- CSF-1:
-
Colony stimulating factor 1
- CTC:
-
Circulating tumour cell
- CXC:
-
CXC-chemokine ligand
- DIAPH3:
-
Diaphanous homologue 3
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EMT:
-
Epithelial-mesenchymal transition
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular-regulated kinase
- FAK:
-
Focal adhesion kinase
- FAS:
-
FS-7-associated surface antigen
- HGF:
-
Hepatocyte growth factor
- IGF:
-
Insulin-like growth factor
- LC3:
-
Microtubule-associated protein 1 light chain 3
- MAPK:
-
Mitogen-activated protein kinase
- MET:
-
Mesenchymal-epithelial transition
- MHC:
-
Major histocompatibility complex
- MICA/B:
-
MHC-I polypeptide-related sequence A/B
- MLCK:
-
Myosin light chain kinase
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mechanistic target of rapamycin
- NF-κB:
-
Nuclear factor kappa B
- NK:
-
Natural killer
- NKG2D:
-
Natural killer group 2D
- NRF2:
-
NF-E2-related factor 2
- PAR:
-
Protease-activated receptor
- PERK:
-
Protein kinase R-like ER kinase
- PI3K:
-
Phosphoinositide 3 kinase
- PTEN:
-
Phosphate and tensin homolog
- ROCK:
-
RhoA-Rho-associated protein kinase
- ROS:
-
Reactive oxygen species
- TAM:
-
Tumour-associated macrophage
- TGFβ:
-
Transforming growth factor β
- TKI:
-
Tyrosine kinase inhibitor
- TME:
-
Tumour microenvironment
- TNF:
-
Tumour necrosis factor
- uPA:
-
Urokinase-type plasminogen activator
- VCAM:
-
Vascular cell adhesion molecule
- VEGF:
-
Vascular endothelial growth factor
- ZEB1:
-
Zinc finger E-box binding homeobox 1
References
van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728(1–2):23–34. https://doi.org/10.1016/j.mrrev.2011.05.002
Hapach LA, Mosier JA, Wang W, Reinhart-King CA (2019) Engineered models to parse apart the metastatic cascade. Npj Precis Oncol 3(1):20. https://doi.org/10.1038/s41698-019-0092-3
Mowers EE, Sharifi MN, Macleod KF (2017) Autophagy in cancer metastasis. Oncogene 36(12):1619–1630. https://doi.org/10.1038/onc.2016.333
Pachmayr E, Treese C, Stein U (2017) Underlying mechanisms for distant metastasis−molecular biology. Visc Med 33(1):11–20. https://doi.org/10.1159/000454696
Kular JK, Basu S, Sharma RI (2014) The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng 5:2041731414557112. https://doi.org/10.1177/2041731414557112
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147(5):992–1009. https://doi.org/10.1016/j.cell.2011.11.016
Lu W, Kang Y (2019) Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell 49(3):361–374. https://doi.org/10.1016/j.devcel.2019.04.010
Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen Y-H (2017) Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med 7(1):1. https://doi.org/10.3390/jcm7010001
Ribatti D, Tamma R, Annese T (2020) Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol 13(6):100773–100773. https://doi.org/10.1016/j.tranon.2020.100773
Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4(2):118–132. https://doi.org/10.1038/nrc1276
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. https://doi.org/10.1016/j.cell.2009.11.007
Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T (2004) Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer 108(2):321–326. https://doi.org/10.1002/ijc.11522
Kolligs FT, Bommer G, Göke B (2002) Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Dig 66(3):131–144. https://doi.org/10.1159/000066755
Li YJ, Wei ZM, Meng YX, Ji XR (2005) Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol 11(14):2117–2123. https://doi.org/10.3748/wjg.v11.i14.2117
Zucker S, Vacirca J (2004) Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 23(1–2):101–117. https://doi.org/10.1023/a:1025867130437
Thiery JP (2002) Epithelial−mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454. https://doi.org/10.1038/nrc822
Raja R, Pandey A, Kumar P (2020) Epithelial to mesenchymal plasticity: role in cancer progression. Front Biosci-Landmark 25(5):838–873. https://doi.org/10.2741/4837
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2(2):76–83. https://doi.org/10.1038/35000025
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437. https://doi.org/10.1038/nm.3394
Kohli K, Pillarisetty VG, Kim TS (2021) Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. https://doi.org/10.1038/s41417-021-00303-x
Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M (2014) Inflammatory chemokines and metastasis—tracing the accessory. Oncogene 33(25):3217–3224. https://doi.org/10.1038/onc.2013.272
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z (2020) Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11(1):5120. https://doi.org/10.1038/s41467-020-18794-x
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374. https://doi.org/10.1038/nrc1075
Scales TME, Parsons M (2011) Spatial and temporal regulation of integrin signalling during cell migration. Curr Opin Cell Biol 23(5):562–568. https://doi.org/10.1016/j.ceb.2011.05.008
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6(2):154–161. https://doi.org/10.1038/ncb1094
Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD (2003) PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol 162(2):281–291. https://doi.org/10.1083/jcb.200212141
Heikenwalder M, Lorentzen A (2019) The role of polarisation of circulating tumour cells in cancer metastasis. Cell Mol Life Sci 76(19):3765–3781. https://doi.org/10.1007/s00018-019-03169-3
Friedl P, Wolf K (2009) Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev 28(1):129–135. https://doi.org/10.1007/s10555-008-9174-3
Sahai E, Marshall CJ (2002) RHO-GTPases and cancer. Nat Rev Cancer 2(2):133–142. https://doi.org/10.1038/nrc725
Kamai T, Arai K, Tsujii T, Honda M, Yoshida K (2001) Overexpression of RhoA mRNA is associated with advanced stage in testicular germ cell tumour. BJU Int 87(3):227–231. https://doi.org/10.1046/j.1464-410x.2001.02030.x
Fritz G, Just I, Kaina B (1999) Rho GTPases are over-expressed in human tumors. Int J Cancer 81(5):682–687. https://doi.org/10.1002/(sici)1097-0215(19990531)81:5%3c682::aid-ijc2%3e3.0.co;2-b
Chiang SPH, Cabrera RM, Segall JE (2016) Tumor cell intravasation. Am J Physiol Cell Physiol 311(1):C1–C14. https://doi.org/10.1152/ajpcell.00238.2015
van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res/Rev Mutat Res 728(1):23–34. https://doi.org/10.1016/j.mrrev.2011.05.002
Reymond N, d’Água BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13(12):858–870. https://doi.org/10.1038/nrc3628
Zavyalova MV, Denisov EV, Tashireva LA, Savelieva OE, Kaigorodova EV, Krakhmal NV, Perelmuter VM (2019) Intravasation as a key step in cancer metastasis. Biochem (Mosc) 84(7):762–772. https://doi.org/10.1134/s0006297919070071
Guo S, Deng C-X (2018) Effect of stromal cells in tumor microenvironment on metastasis initiation. Int J Biol Sci 14(14):2083–2093. https://doi.org/10.7150/ijbs.25720
Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, Qian B-Z, Stanley ER, Cox D, Pollard JW, Muller WJ, Condeelis J, Segall JE (2012) Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res 14(1):R23. https://doi.org/10.1186/bcr3108
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56(4):549–580. https://doi.org/10.1124/pr.56.4.3
Drabsch Y, ten Dijke P (2011) TGF-β signaling in breast cancer cell invasion and bone metastasis. J Mammary Gland Biol Neoplasia 16(2):97–108. https://doi.org/10.1007/s10911-011-9217-1
Fröhlich C, Klitgaard M, Noer JB, Kotzsch A, Nehammer C, Kronqvist P, Berthelsen J, Blobel C, Kveiborg M, Albrechtsen R, Wewer UM (2013) ADAM12 is expressed in the tumour vasculature and mediates ectodomain shedding of several membrane-anchored endothelial proteins. Biochem J 452(1):97–109. https://doi.org/10.1042/bj20121558
Chen J, Imanaka N, Chen J, Griffin JD (2010) Hypoxia potentiates notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer 102(2):351–360. https://doi.org/10.1038/sj.bjc.6605486
Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, Itoh K, Yoshioka K, Kakizaki F, Kitamura T, Oshima M, Taketo MM (2011) Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell 19(1):125–137. https://doi.org/10.1016/j.ccr.2010.11.008
Mekkawy AH, Pourgholami MH, Morris DL (2014) Involvement of urokinase-type plasminogen activator system in cancer: an overview. Med Res Rev 34(5):918–956. https://doi.org/10.1002/med.21308
Ossowski L (1988) Plasminogen activator dependent pathways in the dissemination of human tumor cells in the chick embryo. Cell 52(3):321–328. https://doi.org/10.1016/s0092-8674(88)80025-4
Casar B, Rimann I, Kato H, Shattil SJ, Quigley JP, Deryugina EI (2014) In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated β1 integrin and induction of FAK/PI3K/Akt motility signaling. Oncogene 33(2):255–268. https://doi.org/10.1038/onc.2012.547
Deryugina EI, Kiosses WB (2017) Intratumoral cancer cell intravasation can occur independent of invasion into the adjacent stroma. Cell Rep 19(3):601–616. https://doi.org/10.1016/j.celrep.2017.03.064
Dalal PJ, Muller WA, Sullivan DP (2020) Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol 190(3):535–542. https://doi.org/10.1016/j.ajpath.2019.11.004
Khuon S, Liang L, Dettman RW, Sporn PHS, Wysolmerski RB, Chew T-L (2010) Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci 123(Pt 3):431–440. https://doi.org/10.1242/jcs.053793
Strilic B, Offermanns S (2017) Intravascular survival and extravasation of tumor cells. Cancer Cell 32(3):282–293. https://doi.org/10.1016/j.ccell.2017.07.001
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5(1):28. https://doi.org/10.1038/s41392-020-0134-x
Lou X-L, Sun J, Gong S-Q, Yu X-F, Gong R, Deng H (2015) Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res 27(5):450–460. https://doi.org/10.3978/j.issn.1000-9604.2015.04.10
Leblanc R, Peyruchaud O (2016) Metastasis: new functional implications of platelets and megakaryocytes. Blood 128(1):24–31. https://doi.org/10.1182/blood-2016-01-636399
Hamidi H, Ivaska J (2018) Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18(9):533–548. https://doi.org/10.1038/s41568-018-0038-z
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta (BBA)—Mol Cell Res 1833(12):3481−3498. https://doi.org/10.1016/j.bbamcr.2013.06.026
Kim Y-N, Koo KH, Sung JY, Yun U-J, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012:306879. https://doi.org/10.1155/2012/306879
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24(50):7455–7464. https://doi.org/10.1038/sj.onc.1209085
Valentinis B, Morrione A, Peruzzi F, Prisco M, Reiss K, Baserga R (1999) Anti-apoptotic signaling of the IGF-I receptor in fibroblasts following loss of matrix adhesion. Oncogene 18(10):1827–1836. https://doi.org/10.1038/sj.onc.1202471
Zhong X, Zhang H, Zhu Y, Liang Y, Yuan Z, Li J, Li J, Li X, Jia Y, He T, Zhu J, Sun Y, Jiang W, Zhang H, Wang C, Ke Z (2020) Circulating tumor cells in cancer patients: developments and clinical applications for immunotherapy. Mol Cancer 19(1):15. https://doi.org/10.1186/s12943-020-1141-9
Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Fürst D, Mytilineos J, Kalthoff H, Janssen O, Oberg H-H, Kabelitz D (2013) Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer 133(7):1557–1566. https://doi.org/10.1002/ijc.28174
Gordon N, Kleinerman ES (2009) The role of Fas/FasL in the metastatic potential of osteosarcoma and targeting this pathway for the treatment of osteosarcoma lung metastases. Cancer Treat Res 152:497–508. https://doi.org/10.1007/978-1-4419-0284-9_29
Massagué J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529(7586):298–306. https://doi.org/10.1038/nature17038
Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL (2004) Rolling of human bone−metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Can Res 64(15):5261. https://doi.org/10.1158/0008-5472.CAN-04-0691
Tözeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW (1995) E-selectin-mediated dynamic interactions of breast-and colon-cancer cells with endothelial-cell monolayers. Int J Cancer 60(3):426–431. https://doi.org/10.1002/ijc.2910600326
Krause T, Turner GA (1999) Are selectins involved in metastasis? Clin Exp Metastasis 17(3):183–192. https://doi.org/10.1023/a:1006626500852
Laferrière J, Houle F, Huot J (2004) Adhesion of HT-29 colon carcinoma cells to endothelial cells requires sequential events involving E-selectin and integrin beta4. Clin Exp Metastasis 21(3):257–264. https://doi.org/10.1023/b:clin.0000037708.09420.9a
Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R (1993) Contribution of carbohydrate antigens sialyl lewis A and sialyl lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res 53(2):354–361
Mattila P, Majuri ML, Renkonen R (1992) VLA-4 integrin on sarcoma cell lines recognizes endothelial VCAM-1. Differential regulation of the VLA-4 avidity on various sarcoma cell lines. Int J Cancer 52(6):918−923. https://doi.org/10.1002/ijc.2910520615
Steinbach F, Tanabe K, Alexander J, Edinger M, Tubbs R, Brenner W, Stöckle M, Novick AC, Klein EA (1996) The influence of cytokines on the adhesion of renal cancer cells to endothelium. J Urol 155(2):743–748
Taichman DB, Cybulsky MI, Djaffar I, Longenecker BM, Teixidó J, Rice GE, Aruffo A, Bevilacqua MP (1991) Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul 2(5):347–355. https://doi.org/10.1091/mbc.2.5.347
Tomita Y, Saito T, Saito K, Oite T, Shimizu F, Sato S (1995) Possible significance of VLA-4 (alpha 4 beta 1) for hematogenous metastasis of renal-cell cancer. Int J Cancer 60(6):753–758. https://doi.org/10.1002/ijc.2910600604
Klemke M, Weschenfelder T, Konstandin MH, Samstag Y (2007) High affinity interaction of integrin alpha4beta1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol 212(2):368–374. https://doi.org/10.1002/jcp.21029
Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC, Pasparakis M, Offermanns S (2016) Tumour−cell−induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536(7615):215–218. https://doi.org/10.1038/nature19076
Schumacher D, Strilic B, Sivaraj Kishor K, Wettschureck N, Offermanns S (2013) Platelet-derived nucleotides promote tumor−cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24(1):130–137. https://doi.org/10.1016/j.ccr.2013.05.008
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation establishment and growth. PLoS ONE 4(8):e6562. https://doi.org/10.1371/journal.pone.0006562
Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast−tumour metastasis. Nature 475(7355):222–225. https://doi.org/10.1038/nature10138
Tremblay PL, Auger FA, Huot J (2006) Regulation of transendothelial migration of colon cancer cells by E−selectin−mediated activation of p38 and ERK MAP kinases. Oncogene 25(50):6563–6573. https://doi.org/10.1038/sj.onc.1209664
Cain RJ, Vanhaesebroeck B, Ridley AJ (2010) The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol 188(6):863–876. https://doi.org/10.1083/jcb.200907135
Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH (2006) Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett 580(17):4252–4260. https://doi.org/10.1016/j.febslet.2006.06.056
Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA (2012) The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2(8):706–721. https://doi.org/10.1158/2159-8290.Cd-11-0239
Akhtar M, Haider A, Rashid S, Al-Nabet ADMH (2019) Paget’s “Seed and Soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol 26(1)
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4):274–284. https://doi.org/10.1038/nrc2622
Xie H-Y, Shao Z-M, Li D-Q (2017) Tumor microenvironment: driving forces and potential therapeutic targets for breast cancer metastasis. Chin J Cancer 36(1):36. https://doi.org/10.1186/s40880-017-0202-y
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56. https://doi.org/10.1038/35065016
Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16(11):2927. https://doi.org/10.1158/1078-0432.CCR-09-2329
Smith HA, Kang Y (2017) Determinants of organotropic metastasis. Annu Rev Cancer Biol 1(1):403–423. https://doi.org/10.1146/annurev-cancerbio-041916-064715
Hua H, Kong Q, Yin J, Zhang J, Jiang Y (2020) Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol 13(1):64. https://doi.org/10.1186/s13045-020-00904-3
Fouad YA, Aanei C (2017) Revisiting the hallmarks of cancer. Am J Cancer Res 7(5):1016–1036
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9(1):19. https://doi.org/10.1186/s13578-019-0282-2
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jørgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578):329–335. https://doi.org/10.1038/nature15756
Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R (2011) VEGF-A and Tenascin-C produced by S100A4<sup>+</sup> stromal cells are important for metastatic colonization. Proc Natl Acad Sci 108(38):16002. https://doi.org/10.1073/pnas.1109493108
Chen H, Chengalvala V, Hu H, Sun D (2021) Tumor-derived exosomes: nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B 11(8):2136–2149. https://doi.org/10.1016/j.apsb.2021.04.012
Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7(11):834–846. https://doi.org/10.1038/nrc2256
Klein CA (2020) Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer 20(11):681–694. https://doi.org/10.1038/s41568-020-00300-6
Klein CA (2011) Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev 21(1):42–49. https://doi.org/10.1016/j.gde.2010.10.011
Yao D, Dai C, Peng S (2011) Mechanism of the mesenchymal−epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 9(12):1608–1620. https://doi.org/10.1158/1541-7786.Mcr-10-0568
Yates CC, Shepard CR, Stolz DB, Wells A (2007) Co−culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer 96(8):1246–1252. https://doi.org/10.1038/sj.bjc.6603700
Zhou XD, Agazie YM (2008) Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ 15(6):988–996. https://doi.org/10.1038/cdd.2008.54
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. https://doi.org/10.1038/nrc2621
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292. https://doi.org/10.1016/j.cell.2011.09.024
McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA (2008) Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133(6):994–1005. https://doi.org/10.1016/j.cell.2008.04.045
Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, Fukumura D, Jain RK (2011) C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci U S A 108(1):302–307. https://doi.org/10.1073/pnas.1016917108
Saha S, Panigrahi DP, Patil S, Bhutia SK (2018) Autophagy in health and disease: a comprehensive review. Biomed Pharmacother 104:485–495
Cordani M, Butera G, Pacchiana R, Donadelli M (2017) Molecular interplay between mutant p53 proteins and autophagy incancer cells. Biochim Biophys Acta (BBA)-Rev Cancer 1867(1):19−28
Rybstein MD, Pedro JMB-S, Kroemer G, Galluzzi L (2018) The autophagic network and cancer. Nat Cell Biol 20:243–251
Villar VH, Merhi F, Djavaheri-Mergny M, Durán RV (2015) Glutaminolysis and autophagy in cancer. Autophagy 11(8):1198–1208
Mowers EE, Sharifi MN, Macleod KF (2017) Autophagy in cancer metastasis. Oncogene 36:1619–1630
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564
Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA (2011) PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cel Biol 31(17):3616–3629
Fung C, Lock R, Gao S, Salas E, Debnath J (2008) Induction of autophagy during extracellular matric detachment promotes cell survival. Mol Biol Cell 19:797–806
Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM (2012) Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res 18(2):370–379
Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q (2013) High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol 30:475
Lazova R, Klump V, Pawelek J (2010) Autophagy in cutaneous malignant melanoma. J Cutan Pathol 37(2):256–268
Han C, Sun B, Wang W, Cai W, Lou D, Sun Y, Zhao X (2011) Overexpression of microtubule-associated protein-1 light chain 3 is associated with melanoma metastasis and vasculogenic mimicry. Tohoku J Exp Med 223(4):243–251
Peng Y-F, Shi Y-H, Ding Z-B, Ke A-W, Gu C-Y, Hui B, Zhou J, Qiu S-J, Dai Z, Fan J (2013) Autophagy inhibition suppresses pulmonary metastasis of HCC in mive via impairing anoikis resistance and colonization of HCC cells. Autophagy 9(12):2056–2068
Peng Y-F, Shi Y-H, Shen Y-H, Ding Z-B, Ke A-W, Zhou J, Qiu S-J, Fan J (2013) Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS ONE 8(9):e74407
Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A, Dinsdale D, Condorelli F, Brandner S, Campanella M, Grose R, Jones C, Salomoni P (2012) The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene 32:699–712
Nieto MA, Huang RY-J, Jackson RA, Thiery JP (2016) EMT: 2016. Cell 166(1):21–45
Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano M, Sugimoto K, Miyazono K (2009) Autophagy is activated by TGF-B and potentiates TGF-B-mediated growth inhibition in human hepatocellular cancer cells. Can Res 69(23):8844–8852
Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q, Xiong J (2013) Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis 34(6):1343–1351
Kim YH, Baek SH, Kim EK, Ha JM, Jin SY, Lee HS, Ha HK, Song SH, Kim SJ, Shin HK, Yong J, Kim D-H, Kim CD, Bae SS (2016) Uncoordinated 51-lile kinase 2 signaling pathway regulates epithelial-mesenchymal transition in A549 lung cancer cells. FEBS Lett 590(9):1365–1374
Qiang L, Zhao B, Ming M, Wang N, He T-C, Hwang S, Thorburn A, He Y-Y (2014) Regulation of cell proliferation and migration by p62 through stabilization of twist1. Proc Natl Acad Sci USA 111(25):9241–9246
Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, Huryk H, Mueller C, Adamo L, Deng J, Petricoin EF III, Pastore L, Zaman S, Menezes G, Mize J, Johal J, Edmiston K, Liotta LA (2010) Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS ONE 5(4):e10240
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA (2011) Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res 13(1):202
McGowan PM, Kirstein JM, Chambers AF (2009) Micrometastatic disease and metastatic outgrowth: clinical issues and experimental approaches. Futur Med 5(7):1083–1098
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kupperwasser C, Bierie B, Weinberg RA (2011) Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA 108(19):7950–7955
Karsli-Uzunbas G, Yanxiang Guo J, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E (2014) Autophagy is required for glucose homestasis and lung tumor maintenance. Cancer Discov 4(8):914–927
Lock R, Kenific CM, Leidal AM, Salas E, Debnath J (2014) Autophagy dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov 4(4):466–479
Kenific CM, Stehbens SJ, Goldsmith J, Leidal AM, Faure N, Ye J, Wittmann T, Debnath J (2016) NBR1 enables autophagy-dependent focal adhesion turnover. J Cell Biol 212(5):577–590
Sharifi MN, Mowers EE, Drake LE, Chris C, Chen H, Zamora M, Mui S, Macleod KF (2016) Autophagy promotes focal adhesion disassembly and cell motility of metastatic tumor cells through the direct interaction of paxillin with LC3. Cell Rep 15(8):1660–1672
Kenific CM, Thorburn A, Debnath J (2010) Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol 22(2):241–245
Fung C, Lock R, Gao S, Salas E, Debnath J (2008) Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell 19(3):797–806
Lock R, Debnath J (2008) Extracellular matrix regulation of autophagy. Curr Opin Cell Biol 20(5):538–588
Rouschop KMA, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Investig 120(1):127–141
Avivar-Valderas A, Bobrovnikova-Marjon E, Diehl JA, Bardeesy N, Debnath J, Aguirre-Ghiso JA (2013) Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene 32(41):4932–4940
Chen N, Debnath J (2013) IkB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy 9(8):1214–1227
Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Isreal A, Baud V, Kroemer G (2010) The IKK complex contributes to the induction of autophagy. EMBO J 29(3):619–631
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specficially regulate the activity of Atg4. EMBO J 26(7):1749–1760
Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion A-C, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquière B, Annaert W, Agostinis P, Carmeliet P (2014) Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26(2):190–206
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited−the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128(11):2527–2535
Kadandale P, Stender JD, Glass CK, Kiger AA (2010) Conserved role for autophagy in Rho1-mediated cortical remodeling and blood cell recruitment. Proc Natl Acad Sci USA 107(23):10502–10507
Macintosh RL, Timpson P, Thorburn J, Anderson KI, Thorburn A, Ryan KM (2012) Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle 11:2022–2029
Sandilands E, Serrels B, McEwan DG, Morton JP, Macagno JP, McLeod K, Stevens C, Brunton VG, Langdon WY, Vidal M, Sansom OJ, Dikic I, Wilkinson S, Frame MC (2012) Autophagic targeting of Src promotes cancer cell survival following reduced FAK signalling. Nat Cell Biol 14:51–60
Kim SI, Na H-J, Ding Y, Wang Z, Lee SJ, Choi ME (2012) Autophagy promotes intracellular degradation of type i collagen induced by transforming growth factor (TGF)-b1. J Biol Chem 287(15):11677–11688
Belaid A, Cerezo M, Chargui A, Corcelle-Termeau E, Pedeutour F, Giuliano S, Ilie M, Rubera I, Tauc M, Barale S, Bertolotto C, Brest P, Vouret-Craviari V, Klionsky DJ, Carle GF, Hofman P, Mograbi B (2013) Autophagy plays a critical role in the degradation of active RHOA, the control of cell cytokinesis and genomic instability. Can Res 73(14):4311–4322
Chan EYW, Kir S, Tooze SA (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282(35):25464–25474
Gurkar AU, Chu K, Raj L, Bouley R, Lee S-H, Kim Y-B, Dunn SE, Mandinova A, Lee SW (2013) Identification of ROCK1 kinase as a critical regulator of Beclin1 mediated autophagy during metabolic stress. Nat Commun 4:2189
Gutierrez MG, Munafó DB, Berón W, Colombo MI (2004) Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 117(13):2687–2697
Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A (2011) The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 10(10):1571–1581
Abbi S, Ueda H, Cooper LA, Zhao J, Christopher R, Guan J-L (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol Biol Cell 13(9):3178–3191
Hara T, Takamura A, Kishi C, Iemura S-i, Natsume T, Guan J-L, Mizushima N (2008) FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181(3):497–510
Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, Liao WS-L, Bast J, Robert C (2008) The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Investig 118(12):3917–3929
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14(9):611–622
Galluzzi L, Pietrocola F, Levine B, Kroemer G (2014) Metabolic control of autophagy. Cell 159(6):1263–1276
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB (2005) Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120(2):237–248
Liang J, Shao SH, Xu Z-X, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB (2007) The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9:218–224
Egan DF, Shackelford DB, Mihaylova MM, Gelino SR, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331(6016):456–461
Lu Z, Yang H, Sutton MN, Yang M, Clarke CH, Liao WS-L, Bast RC (2014) ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a-mediated induction of Rab7. Cell Death Differ 21(8):1275–1289
Ma Y, Galuzzi L, Zitvogel L, Kroemer G (2013) Autophagy and cellular immune responses. Immunity 39(2):211–227
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13:722–737
Zhong Z, Sanchez-Lopez E, Karin M (2016) Autophagy, inflammation and immunity: a troika governing cancer and its treatment. Cell 166(2):288–298
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity´s roles in cancer suppression and promotion. Science 331(6024):1565–1570
Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, Di Virgilio F, Zitvogel L, Kroemer G (2011) Autophagy-dependent anticancer immunre responses induced by chemotherapeutic agents in mice. Science 334(6062):1573–1577
Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penualt-Llorca F, Arnould L, Poillot ML, Arveux P, Delaloge S, Andre F, Zitvogel L, Kroemer G (2016) The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 12(5):864–875
Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, Sigl V, Aumayr K, Schmauss G, Fellner N, Handschuh S, Glösmann M, Pasierbek P, Schlederer M, Resch GP, Ma Y, Popper H, Kenner L, Kroemer G, Penninger JM (2014) A dual role for autophagy in a murine model of lung cancer. Nat Commun 5:3056–3070
Akalay I, Janji B, Hasmim M, Noman MZ, André F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Hung NTY, Thiery JP, Mami-Chouaib F, Chouaib S (2013) Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Can Res 73(8):2418–2427
Wei H, Wei S, Gan B, Peng X, Zou W, Gguan J-L (2011) Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev 25(14):1510–1527
Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, Bleackley RC, Goping IS, Chouaib S, Janji B (2013) Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A 110(43):17450–17455
Tittarelli A, Janji B, van Moer K, Noman MZ, Chouaib S (2015) The selective degradation of synaptic connexin 43 protein by hypoxia-induced autophagy impairs natural killer cell-mediated tumor cell killing. J Biol Chem 290(39):23670–23679
Noman MZ, Janji B, Kaminska B, van Moer K, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F, Chouaib S (2011) Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-Cell activity and promotes regression. Can Res 71(18):5976–5986
Messai Y, Noman MZ, Hasmim M, Janji B, Tittarelli A, Boutet M, Baud V, Viry E, Billot K, Nanbakhsh A, Safta TB, Richon C, Ferlicot S, Donnadieu E, Couve S, Gardie B, Orlanducci F, Albiges L, Thiery J, Olive D, Escudier B, Chouaib S (2014) ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Can Res 74(23):6820–6832
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Wels J, Kaplan RN, Rafii S, Lyden D (2008) Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev 22(5):559–574
Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F (2010) Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 9(12):2423–2433
Baixauli F, López-Otín C, Mittelbrunn M (2014) Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5:403
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30(17):3481–3500
Gupta A, Roy S, Lazar AJF, Wang W-L, McAuliffe JC, Reynoso D, McMahon J, Tagushi T, Floris G, Debiec-Rychter M, Schöffski P, Trent JA, Debnath J, Rubin BP (2010) Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc Natl Acad Sci USA 107(32):12333–14338
Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A (2008) Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Can Res 68(5):1485–1494
Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA (2006) Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Can Res 66(3):1702–1711
Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Investig 117(2):326–336
Acknowledgements
Work in the laboratory of NR is supported by funds from Centre for Cancer Biology, University of South Australia and Neurosurgical Research Foundation (NRF), Adelaide, Australia.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Disclosure of Interests
All authors declare they have no conflict of interest.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Polara, R., van Rinsum, D., Robinson, N. (2023). Autophagy in Cancer Metastasis. In: Shravage, B.V., Turksen, K. (eds) Autophagy in Stem Cell Maintenance and Differentiation. Stem Cell Biology and Regenerative Medicine, vol 73. Springer, Cham. https://doi.org/10.1007/978-3-031-17362-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-17362-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17361-5
Online ISBN: 978-3-031-17362-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)