Abstract
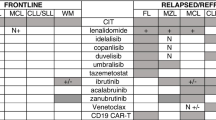
Indolent non-Hodgkin lymphoma (iNHL), including follicular (FL) and marginal zone (MZL) lymphoma, now enjoy durable disease control with first-line immunochemotherapy, with a median overall survival (OS) of over 15 years in most series (Kahl and Yang 2016). However, iNHL is still widely considered incurable in most cases, and disease history remains characterized by a relapsing and remitting course, with each remission period shorter than the previous one, and OS and progression-free survival (PFS) decrease with each subsequent line of conventional therapy (Batlevi et al. 2020). Patients with unmet needs include approximately 20% of FL patients who experience disease progression within 24 months (POD24) after initial chemoimmunotherapy (with a 5-year OS of 48% (Casulo et al. 2015)—although it remains unclear how much this worse outcome is driven by misdiagnosed transformed follicular lymphoma (Freeman et al. 2019)) and those who fail multiple regimens (5-year PFS of 23%) (Rivas-Delgado et al. 2019) have double refractory disease (Gopal et al. 2017) or experience relapse after autologous stem cell transplantation (ASCT) (Sesques et al. 2020). Although promising results were obtained with an immunomodulatory regimen combining anti-C20 Moab and lenalidomide (Leonard et al. 2019; Morschhauser et al. 2019), most current approved therapies do not overcome incremental disease resistance, resulting in multiple lines of treatment with cumulative toxicity over a patient’s lifetime. The autologous anti-CD19 chimeric antigen receptor T cell (CAR-T) therapies tisa-cel and axi-cel, which are now approved for patients with relapsed/refractory (r/r) large B cell lymphoma (LBCL), have also been tested in iNHL, with promising results.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Indolent non-Hodgkin lymphoma (iNHL), including follicular (FL) and marginal zone (MZL) lymphoma, now enjoy durable disease control with first-line immunochemotherapy, with a median overall survival (OS) of over 15 years in most series (Kahl and Yang 2016). However, iNHL is still widely considered incurable in most cases, and disease history remains characterized by a relapsing and remitting course, with each remission period shorter than the previous one, and OS and progression-free survival (PFS) decrease with each subsequent line of conventional therapy (Batlevi et al. 2020). Patients with unmet needs include approximately 20% of FL patients who experience disease progression within 24 months (POD24) after initial chemoimmunotherapy (with a 5-year OS of 48% (Casulo et al. 2015)—although it remains unclear how much this worse outcome is driven by misdiagnosed transformed follicular lymphoma (Freeman et al. 2019)); those who fail multiple regimens (5-year PFS of 23%) (Rivas-Delgado et al. 2019), have double refractory disease (Gopal et al. 2017) or experience relapse after autologous stem cell transplantation (ASCT) (Sesques et al. 2020). Although promising results were obtained with an immunomodulatory regimen combining anti-CD20 Moab and lenalidomide (Leonard et al. 2019; Morschhauser et al. 2019), most current approved therapies do not overcome incremental disease resistance, resulting in multiple lines of treatment with cumulative toxicity over a patient’s lifetime. The autologous anti-CD19 chimeric antigen receptor T cell (CAR-T) therapies tisa-cel and axi-cel, which are now approved for patients with relapsed/refractory (r/r) large B cell lymphoma (LBCL), have also been tested in iNHL, with promising results.
The ZUMA-5 phase 2 trial evaluated the efficacy and safety of axi-cel in 146 patients with r/r iNHL (FL: 124; MZL: 22) after at least two lines of therapy (Jacobson et al. 2020). Among the 104 patients available for the efficacy analysis (84 with FL and 20 with MZL), the overall response rate (ORR) was 92%, with 76% of patients obtaining complete remission (CR). In FL patients, the ORR was 94%, with a CR rate of 80%. Response rates were consistent among patients with high-risk features. With a median follow-up of 17.5 months, 64% of FL patients remained in response. The median duration of response (DoR), PFS, and OS were not reached (Gopal et al. 2017). The safety profile was manageable and appeared favourable in patients with FL compared with that previously reported in LBCL (Neelapu et al. 2017; Locke et al. 2019). Grade ≥ 3 adverse events (AEs) occurred in 126 patients (86%), most commonly neutropenia and infection. Fewer instances of any grade (78%) and high-grade (6%) cytokine release syndrome (CRS) were observed in the FL cohort. The onset of CRS was delayed compared with that seen in LBCL. The event was not resolved in only one patient, who ultimately died due to multiorgan failure (Jacobson et al. 2020). Fifty-six percent of patients experienced neurological events (NEs) of any grade; 15% had grade ≥ 3 events. Most NEs (67/70) resolved by the data cut-off time (Jacobson et al. 2020).
The same reliable results were seen with tisa-cel. In the phase 2 ELARA study, 98 adult patients with r/r FL within 6 months after second or later therapy or that relapsed after ASCT were enrolled (Fowler et al. 2020). Ninety-seven patients received tisa-cel, but 52 were evaluable for efficacy. Unlike the ZUMA-5 trial, bridging therapy was allowed, and 43% of patients received it. Thirty-four of 52 patients (65.4%) achieved a CR, with an ORR of 82.7%. With a median follow-up of 9.9 months, 69% of patients were still in response. Median DoR, PFS, and OS were not reached. Of the 97 patients evaluable for safety (median follow-up of 6.6 months), 69% experienced grade ≥ 3 AEs, most commonly neutropenia; 48% of patients had CRS, but none of them experienced a grade ≥ 3 AE. Any grade NEs occurred in 10% of patients; 2% had a grade ≥ 3 NE, and all recovered. No deaths seen were treatment-related (Fowler et al. 2020).
These preliminary data from the ELARA and ZUMA-5 trials suggest that anti-CD19 CAR-T cell treatment is effective in high-risk or extensively treated r/r iNHL, resulting in a high CR and ORR. Although the benefit/risk ratio seems highly favourable in high-risk FL patients, such as young, double refractory, relapse post-ASCT patients or those with POD24, longer follow-up times are needed to better define the potential for cure and the limited long-term toxicities, especially in view of the emergence of highly efficient competitive therapies, such as bispecific antibodies (Bannerji et al. 2020; Hutchings et al. 2020a,b; Assouline et al. 2020). Data remain scarce in MZL. Clearly, phase III randomized trials are mandatory to confirm the role of CAR-T cells in R/R in NHL, especially in POD24 patients.
-
Anti-CD19 CAR-T cell treatment achieves high CR and ORRs in extensively treated r/r FLs with an acceptable safety profile.
-
The response appears durable, but the median follow-up time remains short.
-
Data remain scarce in MZL.
-
Phase 3 randomized trials are mandatory to confirm the role of CAR-T cells in r/r iNHL, especially in POD24 patients.
References
Assouline SE, Kim WS, Sehn LH, et al. Mosunetuzumab shows promising efficacy in patients with multiply relapsed follicular lymphoma: updated clinical experience from a phase I dose-escalation trial. ASH Congress; 2020, abstract 702.
Bannerji R, Allan JN, Arnason JE, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR-T therapy ASH Congress; 2020, abstract 400.
Batlevi L, Sha F, Alperovich A, et al. Blood. Cancer J. 2020;10(7):74. https://doi.org/10.1038/s41408-020-00340-z.
Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–22. https://doi.org/10.1200/JCO.2014.59.7534. Epub 2015 Jun 29.
Fowler NH, Dickinson M, Dreyling M, et al. Efficacy and safety of tisagenlecleucel in adult patients with relapsed/refractory follicular lymphoma: interim analysis of the phase 2 Elara Trial ASH Congress; 2020, abstract 1149.
Freeman CL, Kridel R, Moccia AA, et al. Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood. 2019;134(9):761–4. https://doi.org/10.1182/blood.2019000258. Epub 2019 Jul 12.
Gopal AK, Kahl BS, Flowers CR, et al. Idelalisib is effective in patients with high-risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood. 2017;129(22):3037–9. https://doi.org/10.1182/blood-2016-12-757740. Epub 2017 Mar 21.
Hutchings M, Mous R, Roost Clausen M, et al. Subcutaneous Epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: updated dose escalation data. ASH Congress 2020a, abstract 402.
Hutchings M, Carlo-Stella C, Bachy E, et al. Glofitamab step-up dosing induces high response rates in patients with hard-to-treat refractory or relapsed non-Hodgkin lymphoma. ASH Congress; 2020b, abstract 403.
Jacobson C, Chavez JC, Sehgal AR, et al. Primary analysis of Zuma-5: a phase 2 study of Axicabtagene Ciloleucel (Axi-Cel) in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). ASH Congress; 2020, abstract700.
Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055–63. https://doi.org/10.1182/blood-2015-11-624288.
Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of Lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37(14):1188–99. https://doi.org/10.1200/JCO.19.00010. Epub 2019 Mar 21.
Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. https://doi.org/10.1016/S1470-2045(18)30864-7. Epub 2018 Dec 2.
Morschhauser F, Le Gouill S, Feugier P, et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol. 2019;6(8):e429–37. https://doi.org/10.1016/S2352-3026(19)30089-4. Epub 2019 Jul 8.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR-T cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447. Epub 2017 Dec 10.
Rivas-Delgado A, Laura Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184(5):753–9. https://doi.org/10.1111/bjh.15708. Epub 2018 Dec 4.
Sesques P, Bourcier J, Golfier C, et al. Clinical characteristics and outcomes of relapsed follicular lymphoma after autologous stem cell transplantation in the rituximab era. Hematol Oncol. 2020;38(2):137–45. https://doi.org/10.1002/hon.2713. Epub 2020 Jan 30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Morschhauser, F., Zinzani, P.L. (2022). Indolent Lymphomas. In: Kröger, N., Gribben, J., Chabannon, C., Yakoub-Agha, I., Einsele, H. (eds) The EBMT/EHA CAR-T Cell Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-94353-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-94353-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94352-3
Online ISBN: 978-3-030-94353-0
eBook Packages: MedicineMedicine (R0)