Abstract
wIRA has been shown to reduce extracellular chlamydial forms and intracellular chlamydial inclusions in different cell culture infection models, and similarly on different human or animal chlamydial species. Repeated wIRA applications increase the efficacy of treatment in vitro, and in vivo in a guinea pig ocular model of inclusion conjunctivitis. The guinea pig model reflects the human ocular disease trachoma, the most common cause of infectious blindness worldwide which is caused by ocular strains of Chlamydia trachomatis. In this model, ocular wIRA treatment reduces conjunctival chlamydial load and ocular pathology. First insights into the mechanisms of anti-chlamydial activity indicate the involvement of both thermal and non-thermal effects. Interestingly, wIRA treatment of non-infected cells renders them more resistant to subsequent chlamydial infection, suggesting cell-related mechanisms that might involve cytochrome C. Further studies envisage the refinement of wIRA treatment protocols, the enhancement of anti-chlamydial activity by adding photodynamic substances, and characterization of the mechanisms underlying the therapeutic benefit of wIRA.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 The Effect of wIRA on Chlamydia
1.1 Introducing Chlamydia
Only few bacterial species can claim to be as famous as the chlamydiae, which gained their notoriety as being a cause of sexually transmitted infections (STI) in humans and as the ‘koala bug’ known to contribute to the extinction of free-living koalas in some regions of Australia [1, 2]. However, these are not the only examples of the Chlamydiaceae family being a threat to both animal and human health. To date, 14 different Chlamydia species have been reported to infect various mammals, birds, and reptiles [2]. A common feature of all these chlamydial species is the obligate intracellular lifestyle involving infection of the cell by the infectious elementary bodies (EBs), and their transformation into reticulate bodies (RBs) inside a vacuole termed inclusion. After several rounds of replication by binary fission, RBs transform back into EBs and exit the cells by extrusion or cell lysis [1]. In contrast to this specialized growth cycle, the range of hosts for individual chlamydial species is highly variable. Whereas Chlamydia (C.) trachomatis almost exclusively infects humans to cause an STI or the chronic eye disease trachoma [1], the ‘koala bug’ is primarily represented by C. pecorum, which is also known to infect ruminants and pigs [2].
Generally, these chlamydial infections are treatable with antibiotics such as azithromycin and tetracycline. However, some chlamydial species have developed resistance strategies, both naturally acquired and in vitro [3]). Therefore, alternative and preferably non-chemical therapy strategies are needed to prevent widespread emergence of antibiotic resistance in the Chlamydiaceae family. Considering these aspects, wIRA is an excellent candidate as a supportive therapy for difficult-to-treat chlamydial infections such as trachoma. Moreover, investigating the effect of wIRA on Chlamydia growth can also serve as a model for the effect of wIRA on other, slow-growing, intracellular bacteria such as Mycobacterium ulcerans [4].
1.2 Establishing an in vitro Model for wIRA Treatment of Chlamydiae
In vitro models are commonly used to determine the effect of new treatments on the viability of bacteria. As a result of their obligate intracellular lifestyle, in vitro models involving Chlamydia require the use of cell cultures for infection, which in turn allows an investigator to assess whether treatment has any negative impact on the cells.
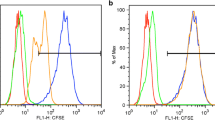
For wIRA treatment in vitro, we use a wIRA radiator with attached light probes allowing treatment of specific cultures in a 24-well plate format (Fig. 21.1a). Initial wIRA-treatment studies used two different cell lines: Vero cells, a permanent cell line derived from African green monkey kidney cells, and HeLa cells, a human cervical cancer cell line. Prior to chlamydial infection, any potential impact of wIRA on the host cells must be evaluated. A resazurin viability assay and expression/activation of various molecular markers of stress and autophagy/apoptosis, specifically the stress kinases Akt, p38, and ERK1/2, the autophagy marker LC-3B, as well as cleaved Caspases 7 and 9 (markers of apoptosis) have consistently shown that wIRA has no negative impact on the viability of either cell line (Fig. 21.1b) regardless of irradiances used ranging from 620 to 3700 W/m2 [5]. This confirms that wIRA does not damage in vitro cultured cells upon treatment [5].
Establishing an in vitro irradiation model for Chlamydia. Figure modified from [5]. Water-filtered infrared-A irradiation in combination with visible light inhibits acute chlamydial infection. PLoS One 9. https://doi.org/10.1371/journal.pone.0102239. (a) Shown is the wIRA setup (left panel) with a close-up of the six light probes (right panel). (b) The results of the resazurin assay after 4 h of wIRA treatment on HeLa cells demonstrate that the cell viability is not affected by irradiation (left panel), and stress kinase markers are not activated 0, 15, and 30 min following wIRA irradiation of HeLa cells for 20 min (right panel). (c) The number of inclusions per nucleus was counted following irradiation of EBs for 20 min and compared to the non-irradiated control (left panel). Representative immunofluorescence images are displayed in the right panel: Green = chlamydial inclusions; blue = nuclei of HeLa cells (DAPI staining). (d) Infectious progeny following 20-min irradiation of infected cells at 40 hpi is expressed as inclusion forming units (IFU), represented as % of the non-irradiated control. (e). The effect of three-fold irradiation (20 min at 24, 36, and 40 hpi) is shown on an ultrastructural level by transmission electron microscopy and subsequent determination of the average number of bacteria per chlamydial inclusion comparing the non-irradiated control with wIRA-treated Chlamydia (left panel). Representative images are displayed on the right showing the severe disruption of chlamydial developmental stages within an inclusion
wIRA treatment can be applied either on the infectious, extracellular (EB) chlamydial form or on the intracellular dividing (RB) form within inclusions. Studies have shown that the anti-chlamydial activity of wIRA is effective in both an in vitro animal model consisting of Vero cells infected with the porcine C. pecorum strain 1710S, and in an in vitro human genital model for which HeLa cells are infected with serovar E (genital serovar) of C. trachomatis [5, 6]. Treatment of EBs with wIRA prior to infection strongly reduces the infectivity of chlamydiae in both models (Fig. 21.1c). Furthermore, a single application of wIRA toward the end of the chlamydial lifecycle (40 hours post infection, hpi), when fully developed chlamydial inclusions are present, reduces the amount of infectious progeny by approximately 40% (Fig. 21.1d). This effect is even more pronounced following repeated irradiation over time (24 hpi, 36 hpi, and 40 hpi), resulting in a reduction by almost 75% in the human genital model. Ultrastructural morphology after wIRA treatment reveals a reduction of total bacterial bodies per inclusion as well as severe disruption of the chlamydial developmental stages (Fig. 21.1e) [5].
The anti-chlamydial activity of wIRA is promising and calls for further investigations and elucidation of potential mechanisms. Initial analyses indicate the relevance of thermal effects which might be, at least in part, responsible for the chlamydial reduction [5]. However, experiments in temperature-controlled settings also show that non-thermal effects of wIRA might be equally important for the anti-chlamydial effect [6]. In conclusion, wIRA has an anti-chlamydial effect in vitro that is independent of the infection model and does not compromise host cell health. Furthermore, initial insights into the working mechanism demonstrate that thermal effects do not fully explain the impact of wIRA on the chlamydiae.
2 Insights into wIRA-Induced Anti-Chlamydial Mechanisms in vitro
2.1 Challenges for Elaborating wIRA Mechanisms in Chlamydia Infection Models
Investigating potential mechanisms underpinning the anti-chlamydial effects of wIRA in vitro is challenging: Obligate intracellular bacteria like Chlamydia depend on host cells and their development cycle cannot be studied in cell-free systems. Therefore, the anti-chlamydial impact of wIRA could originate from a prokaryotic and/or eukaryotic response. First, thermal effects have to be separated from non-thermal influences using appropriate experimental settings such as tightly controlled temperature settings (e.g. temperature-controlled water bath, [5, 6]). Furthermore, the wavelength spectrum might impact irradiation results. Irradiation wavelengths ranging from 400 to 1400 nm are more efficient in reducing extracellular chlamydial forms than wavelengths ranging from 780 to 1400 nm [6]. Furthermore, the anti-chlamydial activity of wIRA is “dose-dependent” (higher irradiances are more effective), but independent of the infectious dose [5, 6].
Extracellular chlamydial forms (EBs) can be irradiated before any contact with the cell monolayer by irradiating an EB suspension which is added to the monolayer after irradiation. The anti-chlamydial activity of wIRA in this setting is primarily caused by direct interactions between wIRA and bacterial EB structures. These anti-chlamydial effects of wIRA could be mediated by modifying the EB surface proteins and limiting the potential of EB to enter host cells and form the inclusions which produce the next generation of infectious EBs. Results showing a reduced ratio of inclusions/host cells in irradiated vs. control conditions from [5] favor this hypothesis (Fig. 21.1c). However, specific bacterial target proteins have not been investigated or identified to date.
Alternatively, it is possible that irradiated EBs differentiate less effectively into RBs after entering the host cells and that a reduction in division events induced by unknown mechanisms reduces the number of infectious particles in inclusions.
A single irradiation of infected host cells with wIRA at 40 hpi reduces chlamydial infectivity to 62.9% of controls (≈40% reduction) [5]. At 40 hpi, chlamydial inclusions are matured and primarily contain EBs which can infect new host cells. At this stage, anti-chlamydial effects on bacterial progeny are most likely caused by irradiation damage of EBs within mature inclusions (thereby leading to the same hypotheses as stated above). However, this hypothesis awaits confirmation.
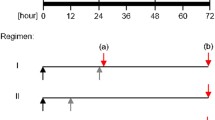
Interestingly, treatment of infected cells with wIRA 24, 36, 40 hpi reduces infectious progeny by 75% compared to approximately 40% following a single treatment at 40 hpi [5]. These findings indicate that biological effects can be increased by repeated treatments and that the full anti-chlamydial efficiency of wIRA is not reached after a single treatment. Irradiation at three time points means that chlamydial inclusions are irradiated at different stages of the development (Fig. 21.2). At 24 hpi, inclusions mainly contain dividing RBs, whereas at later time points, mixtures of RBs and EBs (or mainly EBs) are present. The difference between three wIRA treatments and a single treatment could therefore also arise from different susceptibilities of chlamydial developmental stages, meaning that, for example, growing inclusions are more vulnerable to irradiation than mature inclusions. Transmission electron microscopy analysis of triple-irradiated inclusions have revealed that the ratio of chlamydial development stages does not change upon irradiation, but that the overall number of chlamydial particles within an inclusion are reduced by about 50% [5]. This supports the hypothesis that irradiation of chlamydial inclusions interacts with the efficiency of chlamydial development such as the division of RBs and RB to EB differentiation. Experiments to investigate the most effective time point for wIRA irradiation during the chlamydial life cycle are currently being performed.
wIRA irradiation of host cells and/or different chlamydial developmental stages: Figure modified from [4]. wIRA-hyperthermia as a treatment option for intercellular bacteria, with special focus on Chlamydiae and Mycobacteria. Int J Hyperthermia. 2020; 37(1): 373–383. https://doi.org/10.180/02656736.2020.1751312. PMID: 32319834. Figure 21.2 summarizes the experimental approach to investigate anti-chlamydial wIRA effects during the Chlamydia trachomatis life cycle. wIRA irradiation displays anti-chlamydial activity on EB/RB bacterial stages as well as on host cells/Chlamydia combinations
Surprisingly, irradiation of host cells prior to infection (total of four irradiations at 8, 6, 3, and 0 h prior to infection) is sufficient to reduce subsequent chlamydial infectivity by about 35%, independent of the wavelength spectrum [7]. Furthermore, combining the irradiation of EBs and host cells before infection enhances the anti-chlamydial effects of wIRA and leads to a maximum reduction in subsequent infectivity of 87% [7]. These findings indicate that the effects of irradiation on bacterial and cellular structures must act synergistically. One major limitation in testing all the above-mentioned hypotheses is that anti-chlamydial effects can only be investigated in a combined host-Chlamydia system. Theoretically, irradiated bacterial structures could interact with host cell structures or pathways and irradiated host cells could impact non-irradiated bacteria. This hinders the identification of specific irradiation target structures in bacteria and/or host cells. The future will show, how (and if) the observed anti-chlamydial mechanisms can be investigated in more detail.
2.2 The Impact of Cellular Cytokines on the Working Mechanism of wIRA
Cytokines are inflammatory products secreted by host cells after infection. Chlamydia-infected cells (without irradiation) and irradiated cells (without chlamydial infection) exhibit a similar cytokine secretion pattern, including the secretion of IL-6, IL-8, RANTES, MIF/GIF, and Serpin E1 [5]. This might indicate that chlamydial infection and wIRA irradiation similarly trigger the host immune response and might result in the increased resistance of host cells to a chlamydial infection [7] if they are treated with wIRA prior to infection.
Kuratli et al. [8] investigated the impact of IL-6, IL-8, and RANTES cytokine secretion from HeLa cells during triple wIRA treatment (24, 36, and 40 hpi). Interestingly, impaired cytokine production or secretion does not influence the anti-chlamydial effects of wIRA-irradiation [8], indicating that wIRA-mediated effects are independent of these cytokines.
Inflammatory responses of the host immune system are typically required to clear ongoing infections. However, in the case of Chlamydia, inflammatory responses are known to mediate negative infection sequelae such as pelvic inflammatory disease or ocular scarring in trachoma [1]. For potential future in vivo wIRA applications, the observation that wIRA acts independently from host cell cytokine secretion is of utmost importance as this would indicate that wIRA treatment remains effective, even when the host immune system is impaired. In contrast, treatment of chlamydial infections by antibiotics is hypothesized to negatively impact the development of a protective host immune response, as described by the so-called “arrested immunity hypothesis” [9]. However, the fact that cytokine release by the host cell is not impaired by wIRA irradiation [5] indicates that consequences like the arrested immunity hypothesis after wIRA irradiation are unlikely.
2.3 Other Cellular and Tissue Effects of wIRA
Based on current literature, we conclude that host cell cytokines are unlikely to be involved in the anti-chlamydial effect of wIRA. Future investigations are needed to elucidate alternative potential targets or pathways in the working mechanism. Kuratli and Borel [10] have presented an overview on studies about wIRA or irradiation with similar wavelengths spectra, putting them into the context of potential future treatments of patients with trachoma [10], whereas a recent review specifically discusses the effects of wIRA on obligate intracellular bacteria, namely C. trachomatis and Mycobacterium ulcerans (causing Buruli ulcer, [4]).
The effects of wIRA have been intensively investigated in several clinical applications outside of infectious diseases by other authors (reviewed in [10]). Known in vivo effects include increases in oxygen partial pressure, temperature, and tissue blood flow in the irradiated area. However, the mechanistic pathways at a cellular level which allow physical properties of wIRA irradiation to be “transduced” into biological systems are still not fully understood. The current major hypothesis includes cytochrome c (a mitochondrial membrane protein involved in the respiratory chain) as the primary photo-acceptor which serves as the key molecule for the resulting light action mechanisms [11].
3 wIRA and Chlamydia in the Ocular Model: A Success Story
3.1 Trachoma in Humans: Why Do We Need wIRA?
Blinding trachoma, caused by ocular strains of C. trachomatis, remains the most common cause of infectious blindness worldwide ([12], WHO-Trachoma, http://www.who.int/topics/trachoma/en/) and is a neglected tropical disease which particularly affects children. Trachoma develops as a sequela of recurrent C. trachomatis infections of the conjunctival epithelium triggering fibrosis and scarring of the conjunctiva and leading to trichiasis and corneal opacity with blindness [1]. In the past few years, a number of global health organizations have aimed to eliminate blinding trachoma by the year 2020 (SAFE strategy by the WHO). The SAFE strategy includes surgery for trichiasis, antibiotic treatment (azithromycin as primary frontline antibiotic) for active trachoma, facial cleanliness, as well as environmental improvement [1]. As of January 2020, although 13 countries have achieved elimination goals ([12] WHO-Trachoma, http://www.who.int/topics/trachoma/en/), the disease is not yet eradicated on a global scale, and so far no vaccine is available. The current strategy for treatment and prevention measurements could highly profit from additional non-chemical interventions that limit disease, reduce transmission, and prevent re-infections. wIRA has not only proven its efficacy on mature chlamydial inclusions (disease) but also on extracellular infectious stages, the elementary bodies (EBs), and transmission limitation (re-infection). wIRA radiators are easy-to-use devices that do not require specific environmental conditions or skilled medical personnel; they can even be used in remote areas without a power supply. Their use in remote villages as an ambulant therapy could bring treatments closer to the affected communities. This motivated our group to explore the use of wIRA in trachoma models in vitro and in vivo.
3.2 Preliminary In Vitro Steps
A suitable in vitro model to evaluate the anti-chlamydial effect of wIRA involves human conjunctival epithelial cells (HCjE) and C. trachomatis strain HAR-36, an ocular serotype B [7]. A reduction of more than 50% in fully developed mature chlamydial inclusions (40 hpi) can be achieved using a single wIRA treatment of 30 min (2100 W/m2). This could be theoretically transferred into an in vivo setting which could reduce the chlamydial load in the conjunctiva, at least partially. Repetition of the wIRA treatment in human patients, for example daily, could further reduce the chlamydial load. The wIRA treatment of EBs alone renders them less infectious and might reduce transmission of the extracellular chlamydial stages in the in vivo setting. The lower prevalence of C. trachomatis EBs in the environment might also reduce transmission events and lead to fewer inclusions formed in conjunctival epithelial cells. Even more encouraging are the results of the combined treatment of EBs and HCjE cells with wIRA prior to chlamydial infection. It can be hypothesized that wIRA irradiation of non-infected cells might pre-condition cells to be more resistant to the initial infection or be more effective in subsequent chlamydial clearance. Four times irradiation of HCjE prior to infection (8 h, 6 h, 3 h, and 0 h) reduces the chlamydial burden when non-irradiated EBs are added by around 35% of the control. Infection of irradiated HCjE cells with irradiated EBs results in the most pronounced reduction of chlamydial load (70–87%), thereby suggesting that wIRA protects cells from infection. It would be of great importance to understand the host cell factors that are involved in the anti-chlamydial effect of wIRA. These promising in vitro results encouraged a transition of work into an in vivo setting, the guinea pig model of inclusion conjunctivitis.
3.3 Promising In Vivo Results
The guinea pig model of inclusion conjunctivitis examines the effect of wIRA in a C. caviae conjunctival infection model [13]. The major advantage of this model is that it assesses wIRA in a complex tissue (the conjunctiva) instead of previous in vitro studies on cell monolayers. Chlamydial inclusions are formed in the conjunctival epithelium and inflammatory cells are recruited to the epithelium and underlying stroma after infection. Thus, the effect of wIRA on inflammatory processes can also be studied in this model. As expected, and in accordance with previous in vitro studies, repeated treatment for 30 min each (day 2 and 4 pi, 2100 W/m2) is more effective in reducing chlamydial load and ocular pathology than a single treatment (day 2). Over an observation period of 21 days, wIRA reduces the ocular pathology score (days 7 and 14 pi) and the chlamydial conjunctival load (days 2, 4, 7, and 14 pi). The latter denotes a reduction in infectious EBs, and therefore reflects a reduced re-infection and transmission. At 21 dpi, chlamydial inclusions in conjunctival epithelial cells are less numerous in treated guinea pigs and the acute inflammatory response is dampened (lower number of polymorphonuclear neutrophils), suggesting that wIRA can even reduce inflammation. Of importance, the ocular and systemic immune response normally present following C. caviae infection is not suppressed by wIRA treatment. In conclusion, wIRA might be capable to reducing trachoma transmission and pathology of ocular chlamydial infections in human patients. These promising findings confirm the results of previous in vitro studies [5,6,7,8] and encourage further investigations for the application of wIRA in the field of trachoma therapeutics. In a very recent report [14] possible, other fields of application have been outlined.
3.4 Future Plans and Outlook
wIRA can reduce the chlamydial burden in vitro and in vivo by affecting mature inclusions as well as by reducing infectious EBs. In the future, wIRA treatment of trachoma patients could be applied by irradiation of the C. trachomatis-infected conjunctiva through a closed eyelid, thereby reaching the infected area (inner conjunctival lining) without harming the deeper structures of the eye such as the vitreous body or the retina, or resulting in corneal damage [4]. Ocular safety studies are needed to exclude deleterious effects of wIRA on sensitive ocular structures, such as the cornea, lens, vitreous body, and retina [4]. Moreover, short-term irradiation protocols including various irradiances would be preferable to apply wIRA in the clinical setting. Future in vitro and in vivo studies aim to investigate the daily application of wIRA to further reduce chlamydial load. Of potential benefit would be to shorten the irradiation time from 30 to 10 min while retaining anti-chlamydial efficacy in view of treatment protocols suitable for children, the main age group affected by trachoma. Enhancement of the anti-chlamydial activity might be achieved by combining wIRA with photodynamic substances (e.g., hypericin) as photodynamic therapy (PDT). PDT using Riboflavin and UV-A therapy, called corneal cross-linking (CXL), has been implemented as treatment for keratoconus in human patients and to treat infectious keratitis in animal and human patients [15, 16]. Similar approaches are envisaged for wIRA, but necessitate a step back to elaborate this idea further in the in vitro model.
Abbreviations
- Akt :
-
Stress kinase
- C. :
-
Chlamydia
- CXL:
-
Corneal cross-linking
- dpi:
-
Days post infection
- EBs:
-
Elementary bodies (extracellular, infectious form of Chlamydia)
- ERK1/2:
-
Extracellular signal-regulated kinases 1/2
- HCjE:
-
Human conjunctival epithelial cells
- HeLa :
-
Human cervical cancer cells (adenocarcinoma)
- hpi:
-
hours post infection
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- LC-3B:
-
Autophagy marker
- MIF/GIF:
-
Macrophage migration inhibiting factor (MIF), glucosylation inhibiting factor (GIF)
- PDT:
-
Photodynamic therapy
- pi :
-
post infection
- RBs:
-
Reticulate bodies (intracellular, metabolically active and dividing form of Chlamydia).
- RANTES:
-
Regulated upon activation, normal T Cell expressed, and presumably secreted (also called CCL5 for chemokine [C-C motif] ligand 5)
- SAFE strategy:
-
Acronym for actions in trachoma treatment and elimination programs: Surgery, Antibiotics, Facial Cleanliness, and Environmental improvement
- Serpin E1:
-
Serpin Family E member 1, also known as PAI-1 (plasminogen activator inhibitor 1)
- STI:
-
Sexually transmitted infection
- Vero cells:
-
African green monkey kidney cells
- WHO:
-
World Health Organization
References
Jordan S, Nelson D, Geisler W. Chlamydia trachomatis infections. In: Tan M, Hegeman JH, Sütterlin C, editors. Chlamydia biology: from genome to disease. Norfolk: Caister Academic Press; 2020. p. 1–30.
Sachse K, Borel N. Recent advances in epidemiology, pathology and immunology of veterinary chlamydiae. In: Tan M, Hegeman JH, Sütterlin C, editors. Chlamydia biology: from genome to disease. Norfolk: Caister Academic Press; 2020. p. 403–28.
Sandoz KM, Rockey DD. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010;5:1427–42.
Borel N, Sauer-Durand AM, Hartel M, et al. WIRA: hyperthermia as a treatment option for intracellular bacteria, with special focus on Chlamydiae and mycobacteria. Int J Hyperthermia. 2020;37:373–83.
Marti H, Koschwanez M, Pesch T, et al. Water-filtered infrared A irradiation in combination with visible light inhibits acute chlamydial infection. PLoS One. 2014;9(7):e102239.
Marti H, Blenn C, Borel N. The contribution of temperature, exposure intensity and visible light to the inhibitory effect of irradiation on acute chlamydial infection. J Photochem Photobiol B. 2015;153:324–33.
Rahn C, Marti H, Frohns A, et al. Water-filtered infrared A reduces chlamydial infectivity in vitro without causing ex vivo eye damage in pig and mouse models. J Photochem Photobiol B. 2016;165:340–50.
Kuratli J, Pesch T, Marti H, et al. Water filtered infrared A and visible light (wIRA/VIS) irradiation reduces Chlamydia trachomatis infectivity independent of targeted cytokine inhibition. Front Microbiol. 2018;9:2757.
Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of Chlamydia control. Sex Transm Dis. 2008;4:53–4.
Kuratli J, Borel N. Perspective: water-filtered infrared-A-radiation (wIRA) – novel treatment options for chlamydial infections? Front Microbiol. 2019;10:1053.
Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–58.
WHO. Trachoma. Geneva: WHO; 2020.
Inic-Kanada A, Stojanovic M, Miljkovic R, et al. Water-filtered infrared A and visible light (wIRA/VIS) treatment reduces Chlamydia caviae-induced ocular inflammation and infectious load in a Guinea pig model of inclusion conjunctivitis. J Photochem Photobiol B. 2020;209:111953.
Gazel D, Demirbakan H, Erinmez M. In vitro activity of hyperthermia on swarming motility and antimicrobial susceptibility profiles of Proteus mirabilis isolates. Int J Hyperthermia. 2021;38(1):1002–12. https://doi.org/10.1080/02656736.2021.1943546.
Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60(6):509–23.
Gallhoefer NS, Spiess BM, Guscetti F, et al. Penetration depth of corneal cross-linking with riboflavin and UV-A (CXL) in horses and rabbits. Vet Ophthalmol. 2016;19(4):275–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Kuratli, J., Marti, H., Blenn, C., Borel, N. (2022). Water-Filtered Infrared A (wIRA) Irradiation: Novel Treatment Options for Chlamydial Infections. In: Vaupel, P. (eds) Water-filtered Infrared A (wIRA) Irradiation. Springer, Cham. https://doi.org/10.1007/978-3-030-92880-3_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-92880-3_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92879-7
Online ISBN: 978-3-030-92880-3
eBook Packages: MedicineMedicine (R0)