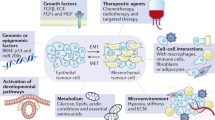
Abstract
The importance of Par-4 in apoptosis has been deciphered in depth. Interestingly, a paradigm shift is emerging with respect to the non-canonical roles of Par-4. The intricacy between Par-4 and EMT is significantly gaining traction, which is the main focus of this chapter. The chapter commences as we first delineate EMT’s transitory and dynamic nature as opposed to the conventional view that portrays EMT as unidirectional and irreversible. We have emphasized EMT’s culpability in the genesis of the metastatic program and how EMT-associated transcription factors (EMT-TFs) manipulate the cancer cells to acquire a motile phenotype suitable for intravasation, migration, and secondary metastasis. We as well discuss the molecular signaling pathways regulating EMT and the challenges rendered by the acquisition of EMT in cancer therapeutics. In the later sections, we have diligently highlighted the emergence of Par-4 as a prospective EMT nullifying candidate and therapeutic opportunities thus evolving around it. Particular emphasis is attributed to novel burgeoning role of Par-4-mediated negative regulation of the following anti-metastatic cascades; for example, modulation of β-catenin pathway, cytoskeletal rearrangements, and extracellular (ECM) remodeling and of course the anti-metastatic microRNAs. Lastly, we put forth innovative insights that link Par-4- and TGF-β-mediated lethal EMT.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Greenburg G, Hay ED (1988) Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Development 102:605–622
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15:117–134
Micalizzi DS, Ford HL (2009) Epithelial–mesenchymal transition in development and cancer. Future Oncol 5:1129–1143
Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829
Batlle E et al (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Cano A et al (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Takkunen M et al (2006) Snail-dependent and-independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J Histochem Cytochem 54:1263–1275
Yee DS et al (2010) The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer 9:162
Potts JD, Runyan RB (1989) Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor β. Dev Biol 134:392–401
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428
Piek E, Moustakas A, Kurisaki A, Heldin C-H, ten Dijke P (1999) TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 112:4557–4568
Moustakas A, Heldin CH (2007) Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 98:1512–1520
Massagué J (2008) TGFβ in cancer. Cell 134:215–230
Heldin C-H, Vanlandewijck M, Moustakas A (2012) Regulation of EMT by TGFβ in cancer. FEBS Lett 586:1959–1970
Ellenrieder V et al (2001) Transforming growth factor β1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res 61:4222–4228
Akiyoshi S et al (1999) C-ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with Smads. J Biol Chem 274:35269–35277
Heldin C-H, Miyazono K, Ten Dijke P (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471
Nishihara A et al (1998) Role of p300, a transcriptional coactivator, in signalling of TGF-β. Genes Cells 3:613–623
Massagué J, Chen Y-G (2000) Controlling TGF-β signaling. Genes Dev 14:627–644
Feng X-H, Derynck R (2005) Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21:659–693
Garg M (2013) Epithelial-mesenchymal transition-activating transcription factors-multifunctional regulators in cancer. World J Stem cells 5:188
Stemmler MP, Eccles RL, Brabletz S, Brabletz T (2019) Non-redundant functions of EMT transcription factors. Nat Cell Biol 21:102–112
Valcourt U, Kowanetz M, Niimi H, Heldin C-H, Moustakas A (2005) TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16:1987–2002
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112:1486–1494
Saika S et al (2004) Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol 164:651–663
Hoot KE et al (2008) Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest 118:2722–2732
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584
Davies M et al (2005) Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-β1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem 95:918–931
Moustakas A, Heldin C-H (2005) Non-Smad TGF-β signals. J Cell Sci 118:3573–3584
Derynck R, Muthusamy BP, Saeteurn KY (2014) Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol 31:56–66
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Xu J, Lamouille S, Derynck R (2009) TGF-β-induced epithelial to mesenchymal transition. Cell Res 19:156–172
Larue L, Bellacosa A (2005) Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24:7443–7454
Conacci-Sorrell M et al (2003) Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of β-catenin signaling, Slug, and MAPK. J Cell Biol 163:847–857
Yook JI, Li X-Y, Ota I, Fearon ER, Weiss SJ (2005) Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem 280:11740–11748
Zhou BP et al (2004) Dual regulation of snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol 6:931–940
Nawshad A, Medici D, Liu C-C, Hay ED (2007) TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci 120:1646–1653
Lucio M (2006) Notch signaling. Clin Cancer Res 12:1074–1079
Wang Z, Li Y, Kong D, Sarkar FH (2010) The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets 11:745–751
Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689
Timmerman LA et al (2004) Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18:99–115
Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP (2004) Integration of TGF-β/Smad and Jagged1/notch signalling in epithelial-to-mesenchymal transition. EMBO J 23:1155–1165
Espinoza I, Pochampally R, Xing F, Watabe K, Miele L (2013) Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther 6:1249
Hu Y-Y, Zheng M-H, Zhang R, Liang Y-M, Han H (2012) Notch signaling in embryology and cancer. Springer, pp 186–198
Wang Z et al (2010) Targeting notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta (BBA)—reviews on Cancer 1806:258–267
Sanchez A et al (2012) p38 MAPK: a mediator of hypoxia-induced cerebrovascular inflammation. J Alzheimers Dis 32:587–597
Imai T et al (2003) Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol 163:1437–1447
Yang M-H et al (2008) Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol 10:295–305
Agani F, Jiang B-H (2013) Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets 13:245–251
Minet E et al (2000) ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 468:53–58
Koong AC, Chen EY, Giaccia AJ (1994) Hypoxia causes the activation of nuclear factor κB through the phosphorylation of IκBα on tyrosine residues. Cancer Res 54:1425–1430
Tam SY, Wu VW, Law HK (2020) Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1α and beyond. Front Oncol 10:486
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196
Lei J et al (2013) Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol Cancer 12:1–11
Li X-L et al (2017) Integrin β4 promotes cell invasion and epithelial-mesenchymal transition through the modulation of slug expression in hepatocellular carcinoma. Sci Rep 7:1–12
Cooper J, Giancotti FG (2019) Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 35:347–367
Soung YH, Clifford JL, Chung J (2010) Crosstalk between integrin and receptor tyrosine kinase signaling in breast carcinoma progression. BMB Rep 43:311–318
Feldkoren B, Hutchinson R, Rapoport Y, Mahajan A, Margulis V (2017) Integrin signaling potentiates transforming growth factor-beta 1 (TGF-β1) dependent down-regulation of E-cadherin expression–important implications for epithelial to mesenchymal transition (EMT) in renal cell carcinoma. Exp Cell Res 355:57–66
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22
Trusolino L, Bertotti A, Comoglio PM (2001) A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell 107:643–654
Abba ML, Patil N, Leupold JH, Allgayer H (2016) MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med 5:8
Wagner S, Ngezahayo A, Murua Escobar H, Nolte I (2014) Role of miRNA let-7 and its major targets in prostate cancer. Biomed Res Int 2014:376326
Sheedy P, Medarova Z (2018) The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res 8:1674
Zhang J, Ma L (2012) MicroRNA control of epithelial–mesenchymal transition and metastasis. Cancer Metastasis Rev 31:653–662
Xu D et al (2011) miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol 193:409–424
Xiao B, Shi X, Bai J (2019) miR-30a regulates the proliferation and invasion of breast cancer cells by targeting snail. Oncol Lett 17:406–413
Nie D, Fu J, Chen H, Cheng J, Fu J (2019) Roles of microRNA-34a in epithelial to mesenchymal transition, competing endogenous RNA sponging and its therapeutic potential. Int J Mol Sci 20:861
Brabletz S, Brabletz T (2010) The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 11:670–677
Nairismägi M-L, Füchtbauer A, Labouriau R, Bramsen JB, Füchtbauer E-M (2013) The proto-oncogene TWIST1 is regulated by microRNAs. PLoS One 8:e66070
Wei F, Cao C, Xu X, Wang J (2015) Diverse functions of miR-373 in cancer. J Transl Med 13:1–8
Si M et al (2007) miR-21-mediated tumor growth. Oncogene 26:2799–2803
Han M et al (2012) Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS One 7:e39520
Wang H et al (2019) microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC cancer 19:738
Wang Y, Li Z, Zhao X, Zuo X, Peng Z (2016) miR-10b promotes invasion by targeting HOXD10 in colorectal cancer. Oncol Lett 12:488–494
Ma L et al (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12:247–256
Gwak JM et al (2014) MicroRNA-9 is associated with epithelial-mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Res Treat 147:39–49
Martello G et al (2010) A MicroRNA targeting dicer for metastasis control. Cell 141:1195–1207
Burk U et al (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9:582–589
Jang K et al (2014) Loss of microRNA-200a expression correlates with tumor progression in breast cancer. Transl Res 163:242–251
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T et al (2011) Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 17:1101–1108
Bae E et al (2014) Definition of smad3 phosphorylation events that affect malignant and metastatic behaviors in breast cancer cells. Cancer Res 74:6139–6149
Hong S et al (2014) SHOX2 is a direct miR-375 target and a novel epithelial-to-mesenchymal transition inducer in breast cancer cells. Neoplasia 16:279–290. e275
Arora H, Qureshi R, Park WY (2013) miR-506 Regulates Epithelial Mesenchymal Transition in Breast Cancer Cell Lines. PLoS One 8(5):e64273
Moes M et al (2012) A novel network integrating a miRNA-203/SNAI1 feedback loop which regulates epithelial to mesenchymal transition. PLoS One 7:e35440
Ding X, Park SI, McCauley LK, Wang CY (2013) Signaling between transforming growth factor β (TGF-β) and transcription factor SNAI2 represses expression of microRNA miR-203 to promote epithelialmesenchymal transition and tumor metastasis. J Biol Chem 288:10241–10253
Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Gotz U, Hermeking H (2011) miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10:4256–4271
Jeanes A, Gottardi C, Yap A (2008) Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27:6920–6929
Kwon MJ (2013) Emerging roles of claudins in human cancer. Int J Mol Sci 14:18148–18180
Salvador E, Burek M, Förster CY (2016) Tight junctions and the tumor microenvironment. Curr Pathobiol Rep 4:135–145
Loh C-Y et al (2019) The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cell 8:1118
Mendez MG, Kojima SI, Goldman RD (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J 24:1838–1851
Scott LE, Weinberg SH, Lemmon CA (2019) Mechanochemical signaling of the extracellular matrix in epithelial-mesenchymal transition. Front Cell Dev Biol 7:135
Wang Y, Shi J, Chai K, Ying X, Zhou PB (2013) The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets 13:963–972
Kang Y, Massagué J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118:277–279
Vandewalle C, Van Roy F, Berx G (2009) The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci 66:773–787
Wu Q, Wang J, Liu Y, Gong X (2019) Epithelial cell adhesion molecule and epithelial-mesenchymal transition are associated with vasculogenic mimicry, poor prognosis, and metastasis of triple negative breast cancer. Int J Clin Exp Pathol 12:1678
Knights AJ, Funnell AP, Crossley M, Pearson RC (2012) Holding tight: cell junctions and cancer spread. Trends Cancer Res 8:61
Berx G, Van Roy F (2009) Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1:a003129
Winter JM et al (2008) Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res 14:412–418
Liu J et al (2016) CDH1 promoter methylation correlates with decreased gene expression and poor prognosis in patients with breast cancer. Oncol Lett 11:2635–2643
Lin X, Shang X, Manorek G, Howell SB (2013) Regulation of the epithelial-mesenchymal transition by claudin-3 and claudin-4. PLoS One 8:e67496
Singh AB, Sharma A, Dhawan P (2010) Claudin family of proteins and cancer: an overview. J Oncol 2010:541957
Porta-De-La-Riva M et al (2011) TFCP2c/LSF/LBP-1c is required for Snail1-induced fibronectin gene expression. Biochem J 435:563–568
Mrozik KM, Blaschuk OW, Cheong CM, Zannettino ACW, Vandyke K (2018) N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 18:939
Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B (1998) Inhibition of β-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci 95:15339–15344
Zhang B et al (2013) Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt–β-catenin signaling. Blood 121:1824–1838
Qian X et al (2014) N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 33:3411–3421
Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A, Georgoulias V, Theodoropoulos PA (2015) Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer 15:399. https://doi.org/10.1186/s12885-015-1386-7
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68:3033–3046
Heerboth S et al (2015) EMT and tumor metastasis. Clin Transl Med 4:6
Ward K et al (2015) Epithelial cell adhesion molecule is expressed in a subset of sarcomas and correlates to the degree of cytological atypia in leiomyosarcomas. Mol Clin Oncol 3:31–36
Soysal SD et al (2013) EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2+, basal-like, and HER2 intrinsic subtypes of breast cancer. Br J Cancer 108:1480–1487
Abd El-Maqsoud NM, Abd El-Rehim DM (2014) Clinicopathologic implications of EpCAM and Sox2 expression in breast cancer. Clin Breast Cancer 14:e1–e9
González B, Denzel S, Mack B, Conrad M, Gires O (2009) EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells 27:1782–1791
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Sedgwick AE, D’Souza-Schorey C (2016) Wnt signaling in cell motility and invasion: drawing parallels between development and cancer. Cancers 8:80
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci 105:6392–6397
Eckert MA et al (2011) Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19:372–386
Murphy DA et al (2011) A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS One 6:e22499
Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE (2012) Hic-5 promotes invadopodia formation and invasion during TGF-β–induced epithelial–mesenchymal transition. J Cell Biol 197:421–437
Slorach EM, Chou J, Werb Z (2011) Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Genes Dev 25:471–484
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Shenoy AK, Lu J (2016) Cancer cells remodel themselves and vasculature to overcome the endothelial barrier. Cancer Lett 380:534–544
Qi J, Wang J, Romanyuk O, Siu C-H (2006) Involvement of Src family kinases in N-cadherin phosphorylation and β-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell 17:1261–1272
Hamidi H, Ivaska J (2018) Every step of the way: integrins in cancer progression and metastasis. Nat Rev Drug Discov 17:31–46
Barthel SR, Gavino JD, Descheny L, Dimitroff CJ (2007) Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets 11:1473–1491
Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD (2009) ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell 20:2207–2217
Gilles C, Newgreen DF, Sato H, Thompson EW (2005) Rise and fall of epithelial phenotype. Springer, pp 297–315
Tsuji T et al (2008) Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 68:10377–10386
Tsuji T, Ibaragi S, Hu G-F (2009) Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res 69:7135–7139
Kim Y-N, Koo KH, Sung JY, Yun U-J, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012:306879
Vachon PH (2011) Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Signal Transduction 2011
Tsai JH, Yang J (2013) Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev 27:2192–2206
Rhim AD et al (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148:349–361
Stegner D, Dütting S, Nieswandt B (2014) Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res 133:S149–S157
Lou X-L et al (2015) Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res 27:450
Kopp H-G, Placke T, Salih HR (2009) Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69:7775–7783
Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L (2012) Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12:239–252
Placke T et al (2012) Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72:440–448
Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12:265–277
Whipple RA et al (2010) Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res 70:8127–8137
Matrone MA et al (2010) Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene 29:3217–3227
Fishbein L et al (2017) Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 31:181–193
Cao Y, Hoeppner LH, Bach S, E G, Guo Y, Wang E et al (2013) Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 Integrin. Cancer Res 73(14):4579-4590
Stoletov K et al (2010) Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci 123:2332–2341
Bonnomet A et al (2012) A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 31:3741–3753
Ocaña OH et al (2012) Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22:709–724
Mejlvang J et al (2007) Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 18:4615–4624
Korpal M et al (2011) Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 17:1101
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Aponte PM, Caicedo A (2017) Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int 2017:5619472
Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH (2013) Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol 61:14.25.11–14.25.14
Rosen JM, Jordan CT (2009) The increasing complexity of the cancer stem cell paradigm. Science 324:1670–1673
Najafi M, Farhood B, Mortezaee K (2019) Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol 234:8381–8395
Jaggupilli A, Elkord E (2012) Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol 2012
Chang C-J et al (2011) p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323
Burikhanov R et al (2009) The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 138:377–388
Wang G et al (2012) Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4) potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 287:21384–21395
Srinivasan S, Ranga RS, Burikhanov R, Han S-S, Chendil D (2007) Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 67:246–253
Gurumurthy S, Goswami A, Vasudevan KM, Rangnekar VM (2005) Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol Cell Biol 25:1146–1161
Rasool RU et al (2016) A journey beyond apoptosis: new enigma of controlling metastasis by pro-apoptotic Par-4. Clin Exp Metastasis 33:757–764
Zhao Y et al (2007) Cancer resistance in transgenic mice expressing the SAC module of Par-4. Cancer Res 67:9276–9285
El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar VM (2003) Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol 23:5516–5525
Park MH, Hong JT (2016) Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cell 5:15
Saegusa M, Hashimura M, Kuwata T, Okayasu I (2010) Transcriptional regulation of pro-apoptotic Par-4 by NF-κB/p65 and its function in controlling cell kinetics during early events in endometrial tumourigenesis. J Pathol 221:26–36
Zhao Y, Rangnekar VM (2008) Apoptosis and tumor resistance conferred by Par-4. Cancer Biol Ther 7:1867–1874
Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta (BBA)-Mol Cell Res 1813:1978–1986
Joshi J et al (2008) Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. EMBO J 27:2181–2193
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Chaudhry P et al (2014) Prostate apoptosis response-4 mediates TGF-β-induced epithelial-to-mesenchymal transition. Cell Death Dis 5:e1044–e1044
Lin A, Karin M Seminars in cancer biology. Elsevier, pp 107–114
Hebbar N, Wang C, Rangnekar VM (2012) Mechanisms of apoptosis by the tumor suppressor Par-4. J Cell Physiol 227:3715–3721
Nalla AK, Gorantla B, Gondi CS, Lakka SS, Rao JS (2010) Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther 17:599–613
Rah B et al (2012) A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS One 7:e44039
Chaudhry P, Singh M, Parent S, Asselin E (2012) Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation. Mol Cell Biol 32:826–839
Ur Rasool R et al (2016) Dual modulation of Ras-Mnk and PI3K-AKT-mTOR pathways: a novel c-FLIP inhibitory mechanism of 3-AWA mediated translational attenuation through dephosphorylation of eIF4E. Sci Rep 6:18800
Pires BR et al (2017) NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS One 12:e0169622
Amin H et al (2016) Par-4 dependent modulation of cellular β-catenin by medicinal plant natural product derivative 3-azido Withaferin A. Mol Carcinog 55:864–881
Qin Q, Xu Y, He T, Qin C, Xu J (2012) Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res 22:90–106
Yang J, Mani SA, Weinberg RA (2006) Exploring a new twist on tumor metastasis. Cancer Res 66:4549–4552
Zhao Z, Rahman MA, Chen ZG, Shin DM (2017) Multiple biological functions of Twist1 in various cancers. Oncotarget 8:20380
Katoch A et al (2020) Overlapping targets exist between the Par-4 and miR-200c axis which regulate EMT and proliferation of pancreatic cancer cells. Transl Oncol 14:100879
Katoch A et al (2018) Dual role of Par-4 in abrogation of EMT and switching on mesenchymal to epithelial transition (MET) in metastatic pancreatic cancer cells. Mol Carcinog 57:1102–1115
Vichalkovski A, Gresko E, Hess D, Restuccia D, Hemmings B (2010) PKB/AKT phosphorylation of the transcription factor Twist-1 at Ser42 inhibits p53 activity in response to DNA damage. Oncogene 29:3554–3565
Pham CG et al (2007) Upregulation of Twist-1 by NF-κB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol 27:3920–3935
Li C-W et al (2012) Epithelial–mesenchymal transition induced by TNF-α requires NF-κB–mediated transcriptional upregulation of Twist1. Cancer Res 72:1290–1300
Burikhanov R et al (2014) Arylquins target vimentin to trigger Par-4 secretion for tumor cell apoptosis. Nat Chem Biol 10:924–926
Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through β-catenin. Science 296:1644–1646
Beurel E, Grieco SF, Jope RS (2015) Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 148:114–131
David CJ et al (2016) TGF-β tumor suppression through a lethal EMT. Cell 164:1015–1030
Mohd Faheem M et al (2020) Par-4 mediated Smad4 induction in PDAC cells restores canonical TGF-β/Smad4 axis driving the cells towards lethal EMT. Eur J Cell Biol 99:151076
Marino N, Marshall J-C, Steeg PS (2011) Protein–protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1. Naunyn Schmiedeberg's Arch Pharmacol 384:351–362
Jung H, Seong H-A, Ha H (2007) NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J Biol Chem 282:35293–35307
Seong H-A, Jung H, Ha H (2007) NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-β (TGF-β) receptor-interacting protein, and negatively regulates TGF-β signaling. J Biol Chem 282:12075–12096
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM (2015) TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res 3:15005
Buijs JT et al (2007) TGF-β and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis 24:609–617
Macı́as-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL (1998) Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem 273:25628–25636
Fukuda T et al (2009) Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284:7149–7156
Vannini I, Fanini F, Fabbri M (2018) Emerging roles of microRNAs in cancer. Curr Opin Genet Dev 48:128–133
Lou W et al (2017) MicroRNAs in cancer metastasis and angiogenesis. Oncotarget 8:115787
Lu D, Tang L, Zhuang Y, Zhao P (2018) miR-17-3P regulates the proliferation and survival of colon cancer cells by targeting Par4. Mol Med Rep 17:618–623
Paterson EL et al (2013) Down-regulation of the miRNA-200 family at the invasive front of colorectal cancers with degraded basement membrane indicates EMT is involved in cancer progression. Neoplasia 15:180–IN122
Korpal M, Kang Y (2008) The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol 5:115–119
Hur K et al (2013) MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 62:1315–1326
Bai WD et al (2014) MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer 135:1356–1368
Mutlu M et al (2016) miR-200c: a versatile watchdog in cancer progression, EMT, and drug resistance. J Mol Med 94:629–644
Hüsemann Y et al (2008) Systemic spread is an early step in breast cancer. Cancer Cell 13:58–68
Ansieau S et al (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14:79–89
Valsesia-Wittmann S et al (2004) Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell 6:625–630
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faheem, M.M., Katoch, A., Goswami, A. (2021). Role of Par-4 in EMT. In: Rangnekar, V.M. (eds) Tumor Suppressor Par-4. Springer, Cham. https://doi.org/10.1007/978-3-030-80558-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-80558-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80557-9
Online ISBN: 978-3-030-80558-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)