Abstract
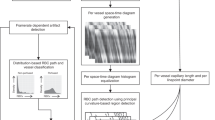
Leukocyte recruitment and adhesion to the endothelial wall are hallmarks of systemic inflammation that occur during several conditions, such as sepsis, ischemia/reperfusion injury and cardiac surgery. Monitoring of microcirculatory leukocytes is a potential candidate tool to assess the inflammatory and pathophysiological status of the patient at the bedside. In the last few years, new methodologies have been introduced using hand-held vital microscopy to monitor microcirculatory leukocytes in surgical and critically ill patients. These methods have been validated to assess microcirculatory leukocyte kinetics at the bedside of the patient. The three methods applied by hand-held vital microscopy to identify leukocytes are known as the conventional manual counting method, the frame averaging method and the space-time diagram method. The conventional manual counting method is accomplished by visual counting of rolling leukocytes. This method cannot distinguish between microcirculatory leukocytes and plasma gaps. The frame averaging method has been developed to distinguish between rolling leukocytes and plasma gaps. This method transforms and filters microcirculatory video-clips in such a manner that red blood cells (RBCs), plasma gaps and non-rolling leukocytes can be distinguished thereby allowing quantification of the number rolling leukocytes. The space-time diagram was developed to measure the velocity of leukocytes and to distinguish and quantify the number of rolling, non-rolling leukocytes in the microcirculation. All three methods have shown increased microcirculatory leukocytes in states of systemic inflammation during surgery and critical illness enabling integration with conventional microhemodynamic assessment using hand-held vital microscopy for point-of-care use at the bedside.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Granger DN, Senchenkova E. Inflammation and the microcirculation. integrated systems physiology—from cell to function. San Rafael: Morgan & Claypool Life Sciences; 2010.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75.
Kubes P, Ward PA. Leukocyte recruitment and the acute inflammatory response. Brain Pathol. 2000;10:127–35.
Gavins FN, Chatterjee BE. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J Pharmacol Toxicol Methods. 2004;49:1–14.
Kara A, Akin S, Ince C. The response of the microcirculation to cardiac surgery. Curr Opin Anaesthesiol. 2016;29:85–93.
Kubes P, Kerfoot SM. Leukocyte recruitment in the microcirculation: the rolling paradigm revisited. News Physiol Sci. 2001;16:76–80.
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–31.
De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41:791–9.
Nencioni A, Trzeciak S, Shapiro NI. The microcirculation as a diagnostic and therapeutic target in sepsis. Intern Emerg Med. 2009;4:413–8.
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98. e1–2
Dobbe JG, Streekstra GJ, Atasever B, van Zijderveld R, Ince C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008;46:659–70.
Bauer A, Kofler S, Thiel M, Eifert S, Christ F. Monitoring of the sublingual microcirculation in cardiac surgery using orthogonal polarization spectral imaging: preliminary results. Anesthesiology. 2007;107:939–45.
Gonzalez S, Sackstein R, Anderson RR, Rajadhyaksha M. Real-time evidence of in vivo leukocyte trafficking in human skin by reflectance confocal microscopy. J Invest Dermatol. 2001;117:384–6.
Kirveskari J, Vesaluoma MH, Moilanen JA, Tervo TM, Petroll MW, Linnolahti E, Renkonen R. A novel non-invasive, in vivo technique for the quantification of leukocyte rolling and extravasation at sites of inflammation in human patients. Nat Med. 2001;7:376–9.
Lim LL, Hoang L, Wong T, Planck SR, Ronick MB, Gould RR, et al. Intravital microscopy of leukocyte-endothelial dynamics using the Heidelberg confocal laser microscope in scleritis and allergic conjunctivitis. Mol Vis. 2006;12:1302–5.
Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, et al. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol Rev. 2008;221:163–81.
Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9Suppl 4:S13–9.
Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44(3):281–99.
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999;5:1209–12.
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15:15101–14.
Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. 2015;3:40.
Dekker NAM, van Leeuwen ALI, van Strien WWJ, Majolée J, Szulcek R, Vonk ABA, et al. Microcirculatory perfusion disturbances following cardiac surgery with cardiopulmonary bypass are associated with in vitro endothelial hyperpermeability and increased angiopoietin-2 levels. Crit Care. 2019;23:117.
Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8.
Uz Z, van Gulik TM, Aydemirli MD, Guerci P, Ince Y, Cuppen D, et al. Identification and quantification of human microcirculatory leukocytes using handheld video microscopes at the bedside. J Appl Physiol (1985). 2018;124:1550–7.
Favaron E, Ince C, Hilty MP, Ergin B, van der Zee P, Uz Z, et al. Capillary leukocytes, microaggregates, and the response to hypoxemia in the microcirculation of coronavirus disease 2019 patients. Crit Care Med. 2021;49:661–70.
Fabian-Jessing BK, Massey MJ, Filbin MR, Hou PC, Wang HE, Kirkegaard H, et al. In vivo quantification of rolling and adhered leukocytes in human sepsis. Crit Care. 2018;22:240.
Uz Z, Aykut G, Massey M, Ince Y, Ergin B, Shen L, et al. Leukocyte-endothelium interaction in the sublingual microcirculation of coronary artery bypass grafting patients. J Vasc Res. 2020;57:8–15.
Japee SA, Pittman RN, Ellis CG. Automated method for tracking individual red blood cells within capillaries to compute velocity and oxygen saturation. Microcirculation. 2005;12:507–15.
Hilty MP, Akin S, Boerma C, Donati A, Erdem Ö, Giaccaglia P, et al. Automated algorithm analysis of sublingual microcirculation in an international multicentral database identifies alterations associated with disease and mechanism of resuscitation. Crit Care Med. 2020;48:e864–e75.
Uz Z, Ince C, Shen L, Ergin B, van Gulik TM. Real-time observation of microcirculatory leukocytes in patients undergoing major liver resection. Sci Rep. 2021;11:4563.
Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–91.
Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–7.
Nakagawa NK, Nogueira RA, Correia CJ, et al. Leukocyte-endothelium interactions after hemorrhagic shock/reperfusion and cecal ligation/puncture: an intravital microscopic study in rat mesentery. Shock. 2006;26:180–6.
Uz Z, de Mol BA, van Gulik TM, Ince C. Sublingual microcirculation reveals fluid overload and leukocytosis in a post-cardiac surgery patient. BMJ Case Rep. 2018;2018:bcr2017223681.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Uz, Z., Ince, C., Arbous, M.S. (2021). Observation of Leukocyte Kinetics Using Handheld Vital Microscopes During Surgery and Critical Illness. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2021. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-73231-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-73231-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73230-1
Online ISBN: 978-3-030-73231-8
eBook Packages: MedicineMedicine (R0)