Abstract
The normal pericardium is a bilayer fibroelastic sac: the outer fibrous and the inner serous pericardium. The latter is itself divided into two components; a visceral layer adjacent to the epicardium and a more external parietal layer. When excessive fluid accumulates in this pericardial space or when the pericardium becomes thickened and stiffened, one of three pericardial compressive syndromes may occur; cardiac tamponade, constrictive pericarditis and effusive-constrictive pericarditis. Pericarditis may be the first clinical manifestation of a non-diagnosed cancer. Twelve percent of patients with no known malignancy presenting with acute pericarditis with or without pericardial effusion will subsequently be diagnosed with cancer within 5 years. Pericardial syndrome in the oncology patients may be secondary to cancer, malignancy complications, or to side-effects of cancer-therapy, however, they often remain idiopathic. Echocardiography, a portable, low-cost, and non-irradiating technique is often performed as first line. Other modalities such as computer tomography (CT) and cardiac magnetic resonance imaging (CMR) may also be used in the diagnosis and evaluation of pericardial syndrome.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Moncada R, Baker M, Salina M, Demos T, Churchill R, Love L, et al. Diagnostic role of computed tomography in pericardial heart disease: congenital defects, thickening, neoplasms, and effusions. Am Heart J. 1982;103(2):263–82.
Søgaard KK, Sørensen HT, Smeeth L, Bhaskaran K. Acute pericarditis and cancer risk: a matched cohort study using linked UK primary and secondary care data. J Am Heart Assoc. 2018;7(16):e009428.
Maisch B, Ristic A, Pankuweit S. Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog Cardiovasc Dis. 2010;53(2):157–63.
Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65(6):1456–9.
Imazio M, Colopi M, De Ferrari GM. Pericardial diseases in patients with cancer: contemporary prevalence, management and outcomes. Heart Br Card Soc. 2020;106(8):569–74.
Chang H-M, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. 2017;70(20):2552–65.
Ala CK, Klein AL, Moslehi JJ. cancer treatment-associated pericardial disease: epidemiology, clinical presentation, diagnosis, and management. Curr Cardiol Rep. 2019;21(12):156.
Kelly K, Swords R, Mahalingam D, Padmanabhan S, Giles FJ. Serosal inflammation (pleural and pericardial effusions) related to tyrosine kinase inhibitors. Target Oncol. 2009;4(2):99–105.
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–7.
Hu J-R, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–68.
Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89.
Ning MS, Tang L, Gomez DR, Xu T, Luo Y, Huo J, et al. Incidence and predictors of pericardial effusion after chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99(1):70–9.
Liu J, Mouhayar E, Tarrand JJ, Kontoyiannis DP. Fulminant Cryptococcus neoformans infection with fatal pericardial tamponade in a patient with chronic myelomonocytic leukaemia who was treated with ruxolitinib: Case report and review of fungal pericarditis. Mycoses. 2018;61(4):245–55.
Liu Y-C, Chien S-H, Fan N-W, Hu M-H, Gau J-P, Liu C-J, et al. Risk factors for pericardial effusion in adult patients receiving allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2015;169(5):737–45.
Norkin M, Ratanatharathorn V, Ayash L, Abidi MH, Al-Kadhimi Z, Lum LG, et al. Large pericardial effusion as a complication in adults undergoing SCT. Bone Marrow Transplant. 2011;46(10):1353–6.
Szpakowski N, Desai MY. Radiation-Associated Pericardial Disease. Curr Cardiol Rep. 2019;21(9):97.
Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bergler J, Bogaert J, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2013;26(9):1013–32.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911.
Kim S-H, Song J-M, Jung I-H, Kim M-J, Kang D-H, Song J-K. Initial echocardiographic characteristics of pericardial effusion determine the pericardial complications. Int J Cardiol. 2009;136(2):151–5.
Sagristà-Sauleda J, Angel Ferrer J, Sánchez A, Permanyer-Miralda G, Soler-Soler J. Effusive–constrictive pericarditis. N Engl J Med. 2004;7.
Ntsekhe M, Wiysonge CS, Commerford PJ, Mayosi BM. The prevalence and outcome of effusive constrictive pericarditis : a systematic review of the literature. Cardiovasc J Afr. 2012;23(5):281–5.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2921–64.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease. J Am Soc Echocardiogr. 2013;26(9):965-1012.e15.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29(4):277–314.
Reuss CS, Wilansky SM, Lester SJ, Lusk JL, Grill DE, Oh JK, et al. Using mitral ‘annulus reversus’ to diagnose constrictive pericarditis. Eur J Echocardiogr. 2009;10(3):372–5.
Ha Jong-Won, Oh Jae K., Ling Lieng H., Nishimura Rick A., Seward James B., Tajik A. Jamil. Annulus paradoxus. Circulation. 2001;104(9):976–8.
Ling LH, Oh JK, Tei C, Click RL, Breen JF, Seward JB, et al. Pericardial thickness measured with transesophageal echocardiography: feasibility and potential clinical usefulness. J Am Coll Cardiol. 1997;29(6):1317–23.
Kim KH, Miranda WR, Sinak LJ, Syed FF, Melduni RM, Espinosa RE, et al. Effusive-constrictive pericarditis after pericardiocentesis. JACC Cardiovasc Imaging. 2018;11(4):534–41.
Xu B, Kwon DH, Klein AL. Imaging of the pericardium: a multimodality cardiovascular imaging update. Cardiol Clin. 2017;35(4):491–503.
Murashita T, Schaff HV, Daly RC, Oh JK, Dearani JA, Stulak JM, et al. experience with pericardiectomy for constrictive pericarditis over eight decades. Ann Thorac Surg. 2017;104(3):742–50.
George TJ, Arnaoutakis GJ, Beaty CA, Kilic A, Baumgartner WA, Conte JV. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94(2):445–51.
Greenwood RD, Rosenthal A, Cassady R, Jaffe N, Nadas AS. Constrictive pericarditis in childhood due to mediastinal irradiation. Circulation. 1974;50(5):1033–9.
von Bibra H, Schober K, Jenni R, Busch R, Sebening H, Blömer H. Diagnosis of constrictive pericarditis by pulsed doppler echocardiography of the hepatic vein. Am J Cardiol. 1989;63(7):483–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
21.1 Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 21.1
Localized large pericardial effusion contained echogenic fibrinous strands. Right atrium and right ventricle were significantly compressed. Interventricular septum bulged into left ventricle during inspiration. Collapse of any cardiac chamber, usually the right-sided chambers, typically occurs before clinical hemodynamic failure, when intracavitary pressure reaches its lowest value and transiently falls below pericardial pressure. Duration of chamber collapse is an indicator of severity. Right atrium collapse, when persisting more than one-third of the cardiac cycle, has been described as an almost 100% sensitive and specific sign of clinical cardiac tamponade. The absence of any cardiac chamber collapse has a >90% negative predictive value for clinical cardiac tamponade. Fibrinous pericardial effusion, which contains echogenic fibrinous and frond-like coating strands, is the major risk factor of the development of constrictive pericarditis (PPTX 2431 kb)
Video 21.2
Dilated IVC (>21 mm) without inspiratory collapse. Dilatation of IVC (>21mm in an adult size heart) and hepatic veins and less than a 50% reduction in the diameter of the dilated IVC during inspiration, is a very sensitive sign of cardiac tamponade, although not very specific (PPTX 1144 kb)
Video 21.3
Respiratory interventricular septal shift revealed in parasternal longitudinal axis view (Video 21.3), parasternal short axis view (Video 21.4), and apical 4-chamber view (Video 21.5). However, it should be noted that an inspiratory septal bulge or “bounce” is common, but not specific for cardiac tamponade, as it can also be seen in other conditions associated with pulsus paradoxus, such as chronic obstructive pulmonary disease, and pulmonary embolism (PPTX 1534 kb)
Video 21.4
Respiratory interventricular septal shift revealed in parasternal longitudinal axis view (Video 21.3), parasternal short axis view (Video 21.4), and apical 4-chamber view (Video 21.5). However, it should be noted that an inspiratory septal bulge or “bounce” is common, but not specific for cardiac tamponade, as it can also be seen in other conditions associated with pulsus paradoxus, such as chronic obstructive pulmonary disease, and pulmonary embolism (PPTX 1037 kb)
Video 21.5
Respiratory interventricular septal shift revealed in parasternal longitudinal axis view (Video 21.3), parasternal short axis view (Video 21.4), and apical 4-chamber view (Video 21.5). However, it should be noted that an inspiratory septal bulge or “bounce” is common, but not specific for cardiac tamponade, as it can also be seen in other conditions associated with pulsus paradoxus, such as chronic obstructive pulmonary disease, and pulmonary embolism (PPTX 1622 kb)
Video 21.6
Large pericardial effusion showing collapse of the right ventricle. Collapse of any cardiac chamber, usually the right-sided chambers, typically occurs before clinical hemodynamic failure, when intracavitary pressure reaches its lowest value and transiently falls below pericardial pressure. Duration of chamber collapse is an indicator of severity. Right atrium collapse, when persisting more than one-third of the cardiac cycle, has been described as an almost 100% sensitive and specific sign of clinical cardiac tamponade. The absence of any cardiac chamber collapse has a >90% negative predictive value for clinical cardiac tamponade (PPTX 1441 kb)
Video 21.7
Large pericardial effusion shown in the apical 4-chamber (a) and parasternal long axis (b) (PPTX 4009 kb)
Video 21.8
In the parasternal long-axis (Video 21.8), apical 4-chamber (Video 21.9), and apical 3-chamber (Video 21.10), a significant respirophasic interventricular septal bounce is seen (PPTX 9767 kb)
Video 21.9
In the parasternal long-axis (Video 21.8), apical 4-chamber (Video 21.9), and apical 3-chamber (Video 21.10), a significant respirophasic interventricular septal bounce is seen (PPTX 15035 kb)
Video 21.10
In the parasternal long-axis (Video 21.8), apical 4-chamber (Video 21.9), and apical 3-chamber (Video 21.10), a significant respirophasic interventricular septal bounce is seen (PPTX 14316 kb)
Video 21.11
Parasternal long axis demonstrating severe decreased left ventricular function with poor contraction of the right ventricle. There is at least moderate pericardial effusion seen posterior to the inferolateral basal segment and the pericardium is thickened and echobright. There is a left pleural effusion. There is also early closure of the aortic valve as a consequence of low cardiac output. The anterior mitral leaflet and the aorto-mitral curtain are thickened likely secondary to previous radiation exposure (PPTX 7630 kb)
Video 21.12
Parasternal short axis at the mitral valve level again demonstrating severely decreased left ventricular function with a moderate-to-severe decrease in right ventricular systolic function. The pericardial effusion is seen posterior to the inferior and lateral wall and is heterogenous (likely fibrinous debris) (PPTX 14149 kb)
Video 21.13
Apical 4-chamber showing severe biventricular systolic dysfunction with at least moderate-to-severe biatrial enlargement. There is a circumferential pericardial effusion best seen lateral to the right atrium and ventricle. Importantly, there is no collapse of the right atrium or ventricle. The anterior mitral leaflet and the aorto-mitral curtain are thickened likely secondary to previous radiation exposure (PPTX 13379 kb)
Video 21.14
Close-up of the left ventricle in the apical 4-chamber view. There is an interventricular septal shudder and bounce (PPTX 12305 kb)
Video 21.15
Apical 4-chamber showing severe biventricular systolic dysfunction with at least moderate-to-severe biatrial enlargement. The pericardial effusion is best seen lateral to the right atrium and ventricle. Importantly, there is no collapse of the right atrium or ventricle. There are an interventricular septal shudder and intermittent bounce (PPTX 10856 kb)
Video 21.16
Off-axis apical 4-chamber showing the pericardial effusion lateral to the left ventricle with sizeable left pleural effusion. The left visceral pericardium adjacent to the left ventricle is thickened and echobright. There is an interventricular septal shudder and bounce (PPTX 13667 kb)
Video 21.17
Plethoric inferior vena cava and hepatic veins with no respiratory changes in the caliber. There is a movement of the probe that may “mimic” collapse of the IVC but this is simply rotation of the probe upward to show the hepatic veins (PPTX 2401 kb)
Video 21.18
1-hour post-pericardiocentesis: In the parasternal long axis (Video 21.18) and the parasternal short axis (Video 21.19), there is no residual pericardial effusion. However, there is a significant respirophasic interventricular septal bounce seen on the second beat in the parasternal long-axis Video and during the first beat in the parasternal short-axis Video (PPTX 2065 kb)
Video 21.19
1-hour post-pericardiocentesis: In the parasternal long axis (Video 21.18) and the parasternal short axis (Video 21.19), there is no residual pericardial effusion. However, there is a significant respirophasic interventricular septal bounce seen on the second beat in the parasternal long-axis Video and during the first beat in the parasternal short-axis Video (PPTX 2141 kb)
Video 21.20
In the apical 4-chamber, there is no more obvious pericardial effusion seen. However, there is a subtle respirophasic interventricular septal bounce visualized (PPTX 2141 kb)
Video 21.21
The inferior vena cava is still dilated and does not collapse during inspiration (PPTX 2169 kb)
Video 21.22
Apical 4-chamber cine of cardiac MRI showing a mild interventricular septal bounce with a small pericardial effusion and a thickened pericardium. There is a moderate right-sided pleural effusion (PPTX 4336 kb)
Video 21.23
A and B are both cardiac MRI cine sequences in short-axis views (different levels in the left ventricle) showing mild interventricular septal bounce. A is a “real-time, free-breathing cine” to try and accentuate the interventricular septal bounce during inspiration and is of inferior image quality. B is a typical cine acquisition with breath holds (PPTX 5377 kb)
Video 21.24
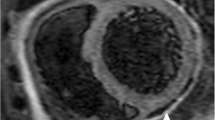
Myocardial tagging sequence of the short axis view showing non-deformation of the grid (adhesion of the myocardium to the pericardium) overlying the right ventricle and the mid-inferior/inferoseptum (see arrows). These signs are consistent with constrictive pericarditis (PPTX 8576 kb)
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lefebvre, B., Kang, Y., Scherrer-Crosbie, M. (2021). Pericardial Effusion, Tamponade, and Constrictive Pericarditis. In: Steingart, R.M., Liu, J.E. (eds) Atlas of Imaging in Cardio-Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-70998-3_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-70998-3_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70997-6
Online ISBN: 978-3-030-70998-3
eBook Packages: MedicineMedicine (R0)