Abstract
Hemodynamics is thought to play an important role in the pathogenesis of cerebral aneurysms and recent development of computer technology makes it possible to simulate blood flow using high-resolution 3D images within several hours. A lot of studies of computational fluid dynamics (CFD) for cerebral aneurysms were reported; therefore, application of CFD for cerebral aneurysms in clinical settings is reviewed in this article.
CFD for cerebral aneurysms using a patient-specific geometry model was first reported in 2003 and it has been revealing that hemodynamics brings a certain contribution to understanding aneurysm pathology, including initiation, growth and rupture. Based on the knowledge of the state-of-the-art techniques, this review treats the decision-making process for using CFD in several clinical settings. We introduce our CFD procedure using digital imaging and communication in medicine (DICOM) datasets of 3D CT angiography or 3D rotational angiography. In addition, we review rupture status, hyperplastic remodeling of aneurysm wall, and recurrence of coiled aneurysms using the hemodynamic parameters such as wall shear stress (WSS), oscillatory shear index (OSI), aneurysmal inflow rate coefficient (AIRC), and residual flow volume (RFV).
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Computational fluid dynamics (CFD) is a computational science which connects experiment and theory. Therefore, it is important to understand the need of validation and verification of CFD for cerebral aneurysms when the hemodynamic results are applied to surgical decision-making in clinical settings.
CFD for a cerebral aneurysm using the patient-specific geometry model was first reported by DA Steinman et al. in 2003 [1], and it has been revealing that hemodynamics contributes to understanding aneurysm pathology including initiation, growth, and rupture [2,3,4,5,6,7,8,9,10]. On the other hand, CFD has not permeated into a clinical setting of cerebral aneurysms due to several limitations including analysis time and complicated process. Therefore, we present our practical application of CFD for the treatment planning of cerebral aneurysms.
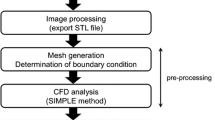
CFD Process for Cerebral Aneurysms
The CFD process for cerebral aneurysms is the following [2, 11, 12]: the digital imaging and communication in medicine (DICOM) datasets of 3D CT angiography and 3D rotational angiography are loaded into Mimics Innovation Suite (Materialise Japan, Kanagawa, Japan) to extract aneurysm geometry as stereolithography (STL). The STL file is integrated into 3-matic (Materialise Japan, Kanagawa, Japan) and geometry in the region of interest is segmented. In addition, the geometry is remeshed by a triangle measuring 0.25 mm at the maximum length for correction of a distortion comprising the STL. The fluid domain is meshed using ANSYS ICEM CFD (ANSYS Inc. Canonsburg, Pennsylvania) to create tetrahedral elements that are established as 0.6 mm at maximum and 0.1 mm at minimum using the Octree’s method. On the geometry surface, six prism layers are added with total heights of 0.148 mm. The inlet is prolonged vertically at the surface to establish fully developed laminar flow according to Poiseuille’s law.
Numerical modeling is performed using ANSYS CFX (ANSYS Inc. Canonsburg, Pennsylvania). For the fluid domain, 3D laminar flow fields are obtained by solving the continuity and Navier-Stokes equations, and discretization is made by the finite volume method. Blood is assumed to be an incompressible Newtonian fluid with a density of 1056 kg/m3 and a viscosity of 0.0035 Pa s. Typical flow waveform of phase-contrast MR imaging is scaled to the inlet and a flow rate is proportional to achieve a physiological wall shear stress (WSS). Traction-free boundary conditions are applied to the outlets. The time steps are 0.0001 s and transient analysis with an initial value specification is performed.
Rupture Status
A lot of CFD studies were reported regarding the rupture status of cerebral aneurysms. Compared with unruptured aneurysms, ruptured aneurysms had significant characteristics such as low WSS [12,13,14,15], high oscillatory shear index (OSI) [12, 14], low aneurysm formation indicator (AFI) [12], prolonged relative residence time (RRT) [16], complex flow pattern, unstable flow pattern [17], and high oscillatory velocity index [18]. Among these hemodynamic parameters, low magnitude of WSS is the most noticeable hemodynamic characteristic associated the rupture status. Since the magnitude of WSS was quantified as time-averaged value, spatial minimum and maximum values on the dome, normalized WSS and low shear area ratio, we should recognize that the distribution of low WSS would be an unchanged observation. However, comparison of the magnitude of WSS in clinical practice should be carried out as an optimal quantification. For instance, time-averaged WSS would be allowed to investigate the rupture status of multiple cerebral aneurysms in a patient with subarachnoid hemorrhage. In particular, mirror aneurysms are a useful disease model to predict the rupture site of an aneurysm. On the other hand, normalized WSS would be recommended for measuring WSS of aneurysms at different locations.
Hyperplastic Remodeling of Aneurysm Wall
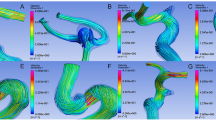
Ku described OSI that was a hemodynamic parameter to evaluate fluctuation of WSS vectors in 1985 [19]. The studies using the pulsatile flow data in a Plexiglas model and intimal plaque thickness in five human carotid bifurcations revealed that oscillations in the direction of wall shear may enhance atherogenesis. Aside from OSI, RRT and AFI can also evaluate oscillation of WSS vectors. These hemodynamic parameters were examined regarding hyperplastic remodeling of aneurysm wall. Since these hemodynamic parameters showed similar distribution to high oscillatory WSS vector (Fig. 1), we can use any of these variables as the preoperative predictor of thick aneurysm wall. In addition, we should recognize that a high OSI region corresponds to a low WSS region.
Comparison of hemodynamic parameters to evaluate the fluctuation of WSS vector in a patient of ruptured middle cerebral artery aneurysm: (a) visualization of OSI, showing high OSI near the neck; (b) visualization of RRT; (c) visualization of AFI, in which low AFI has similar distribution to prolonged RRT and low WSS; (d) visualization of WSS, in which high OSI, prolonged RRT and low AFI regions are depicted in the low WSS area
The prediction of hyperplastic remodeling lesions using CFD [6, 11, 16] would contribute to avoiding intraoperative risks such as inadequate temporary clipping and obstruction of small branches (Fig. 2).
Seventy-six-year-old man with unruptured left internal carotid artery-anterior choroidal artery bifurcation aneurysm: (a, b) visualization of OSI, showing high OSI at the parent artery (white arrow) and the backside of the aneurysm neck at which anterior choroidal artery arises (black arrow); (c) visualization of time-averaged WSS, showing distribution of low WSS (white arrow) which is similar to that of high OSI; (d, e) intraoperative photography, demonstrating atherosclerotic lesions of the parent artery (white arrow) and at the backside of the aneurysm neck (black arrow), respectively, which correspond with high OSI regions; (f) aneurysm clip is applied with an intended space to keep blood flow of the anterior choroidal artery
Recurrence of Coiled Aneurysms
Coil embolization of cerebral aneurysms is widely used; however, recanalization and re-treatment occur more often compared with surgical clipping. Several risk factors for recanalization and re-treatment have been reported, such as a large aneurysm, wide neck width, minor recurrence on cerebral angiogram early after coil embolization, and lower packing density.
A number of authors reported hemodynamic risk factors associated with coiled aneurysms. High WSS observed near the remnant neck of partially coiled aneurysms are more likely to have recanalization [20]. However, since the geometry of neck remnant is obtained only after coiling, it is impossible to predict the recurrence risk as presurgical decision-making based on this method. In a clinical setting, it is desirable that hemodynamic evaluation using presurgical geometry models can predict recanalization of coiled aneurysms. Sugiyama et al. demonstrated the correlations between the hemodynamics before coil embolization and the outcomes after treatment for basilar tip aneurysms [21]. In their study, aneurysmal inflow rate coefficient (AIRC) was calculated by the following equation:
where Qa and Qb were the aneurysmal inflow rate and the basilar artery flow rate, respectively.
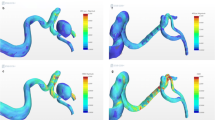
In 57 basilar tip aneurysms, AIRC was significantly higher in the recanalized group and correlated to the types of basilar bifurcation configuration. Although it was sensible to make a decision for coil embolization based on these findings, packing density should be considered simultaneously. Therefore, CFD using porous media modeling was developed to predict recurrence of coil embolization. Umeda et al. calculated residual flow volume (RFV) to quantify the residual aneurysm volume after simulated coiling, which has a mean fluid domain above 1.0 cm/s [22]. In 37 unruptured cerebral aneurysms, the recurrence group had significantly larger RFV than the stable group. Receiver-operating characteristic (ROC) curve analyses showed that the cut-off value of RFV was 20.4 mm3 and that the area under the ROC curve was 0.86. Although this study was retrospective, these findings could be applied to indicate a target packing density. In addition, CFD using double porous media modeling was developed to simulate stent-assisted coiling (Fig. 3) [23]. Since these porous media modeling have discrepancies between actual distribution of placed coil and expanded strut in the aneurysm, large-scale clinical studies are required to confirm the accuracy of the prediction for recurrence of coiled aneurysms.
Seventy-nine-year-old woman with left internal carotid artery-posterior communicating artery bifurcation aneurysm: (a) 3D surface rendering image of rotational angiography; (b) angiography after stent-assisted coiling of the aneurysm with a packing density of 35.9%; (c) head CT revealing subarachnoid hemorrhage 19 months after the coiling; (d) left internal carotid artery angiography revealing the recanalization of the coiled aneurysm; (e) retrospective CFD findings using double porous media setting, one of which is a porous media setting for the coiled aneurysm and another of which is that for an intracranial stent (arrow), to evaluate the hemodynamic changes of stent-assisted coiled aneurysm. Residual flow volume (pink domain) is 62.3 mm3, which is larger than the cut-off value to predict the recurrence of coiled aneurysms
Conclusions
This review describes the practical application of CFD to treatment planning of cerebral aneurysms. Several hemodynamic parameters have valuable aspects not only in an endovascular treatment but also in a direct clipping. Although prospective trials are needed to evaluate the rupture risk of unruptured cerebral aneurysms determined using CFD, recent knowledge of hemodynamics of cerebral aneurysms would enhance the decision-making ability and precision in several clinical settings.
References
Steinman DA, Milner JS, Norley CJ, Lownie SP, Holdsworth DW (2003) Image-based computational simulation of flow dynamics in a giant intracranial aneurysm. AJNR Am J Neuroradiol 24:559–566
Fukazawa K, Ishida F, Umeda Y, Miura Y, Shimosaka S, Matsushima S, Taki W, Suzuki H (2015) Using computational fluid dynamics analysis to characterize local hemodynamic features of middle cerebral artery aneurysm rupture points. World Neurosurg 83:80–86
Kulcsa Z, Marosfo M, Paa G (2011) Hemodynamics of cerebral aneurysm initiation: the role of wall shear stress and spatial wall shear stress gradient. AJNR Am J Neuroradiol 32:587–594
Mantha A, Karmonik C, Benndorf G, Strother C, Metcalfe R (2006) Hemodynamics in a cerebral artery before and after the formation of an aneurysm. AJNR Am J Neuroradiol 27:1113–1118
Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, Kolega J (2007) Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38:1924–1931
Omodaka S, Sugiyama SI, Inoue T, Funamoto K, Fujimura M, Shimizu H, Hayase T, Takahashi A, Tominaga T (2012) Local hemodynamics at the rupture point of cerebral aneurysms determined by computational fluid dynamics analysis. Cerebrovasc Dis 34:121–129
Stress S, Boussel L, Rayz V et al (2008) Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39:2997–3002
Takao H, Murayama Y, Otsuka S, Qian Y, Mohamed A, Masuda S, Yamamoto M, Abe T (2012) Hemodynamic differences between unruptured and ruptured intracranial aneurysms during observation. Stroke 43:1436–1439
Tateshima S, Villablanca JP, Vin F (2011) Wall shear stress distribution inside growing cerebral aneurysm. AJNR Am J Neuroradiol 32:1732–1739
Tsuji M, Ishikawa T, Ishida F, Furukawa K, Miura Y, Sano T, Tanemura H, Umeda Y, Shimosaka S, Suzuki H (2017) Stagnation and complex flow in ruptured cerebral aneurysms: a possible association with hemostatic pattern. J Neurosurg 126:1566–1572
Furukawa K, Ishida F, Tsuji M et al (2018) Hemodynamic characteristics of hyperplastic remodeling lesions in cerebral aneurysms. PLoS One 13:e0191287
Miura Y, Ishida F, Umeda Y, Tanemura H, Suzuki H, Matsushima S, Shimosaka S, Taki W (2013) Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke 44:519–521
Lu G, Huang L, Zhang XL, Wang SZ, Hong Y, Hu Z, Geng DY (2011) Influence of hemodynamic factors on rupture of intracranial aneurysms: patient-specific 3D mirror aneurysms model computational fluid dynamics simulation. Am J Neuroradiol 32:1255–1261
Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, Siddiqui AH, Levy EI, Meng H (2011) Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke 42:144–152
Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S, Huang Q, Liu J (2013) Morphological and hemodynamic analysis of mirror posterior communicating artery aneurysms. PLoS One 8:e55413
Sugiyama S, Niizuma K, Nakayama T, Shimizu H, Endo H, Inoue T, Fujimura M, Ohta M, Takahashi A, Tominaga T (2013) Relative residence time prolongation in intracranial aneurysms: a possible association with atherosclerosis. Neurosurgery 73:767–776
Cebral JR, Mut F, Weir J, Putman C (2011) Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol 32:264–270
Sano T, Ishida F, Tsuji M, Furukawa K, Shimosaka S, Suzuki H (2017) Hemodynamic differences between ruptured and unruptured cerebral aneurysms simultaneously existing in the same location: 2 case reports and proposal of a novel parameter oscillatory velocity index. World Neurosurg 98:868.e5–868.e10
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5:293–302
Luo B, Yang X, Wang S et al (2011) High shear stress and flow velocity in partially occluded aneurysms prone to recanalization. Stroke 42:745–753
Sugiyama S, Niizuma K, Sato K, Rashad S, Kohama M, Endo H, Endo T, Matsumoto Y, Ohta M, Tominaga T (2016) Blood flow into basilar tip aneurysms: a predictor for recanalization after coil embolization. Stroke 47:2541–2547
Umeda Y, Ishida F, Tsuji M, Furukawa K, Shiba M, Yasuda R, Toma N, Sakaida H, Suzuki H (2017) Computational fluid dynamics (CFD) using porous media modeling predicts recurrence after coiling of cerebral aneurysms. PLoS One 12:e0190222
Tsuji M, Ishida F, Kishimoto T, Furukawa K, Miura Y, Shiba M (2016) Computational fluid dynamics using double porous media modeling to simulate stent-assisted coiling of cerebral aneurysm. Proc Neurosci 1:144–147
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this paper
Cite this paper
Ishida, F., Tsuji, M., Tanioka, S., Tanaka, K., Yoshimura, S., Suzuki, H. (2021). Computational Fluid Dynamics for Cerebral Aneurysms in Clinical Settings. In: Esposito, G., Regli, L., Cenzato, M., Kaku, Y., Tanaka, M., Tsukahara, T. (eds) Trends in Cerebrovascular Surgery and Interventions. Acta Neurochirurgica Supplement, vol 132. Springer, Cham. https://doi.org/10.1007/978-3-030-63453-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-63453-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63452-0
Online ISBN: 978-3-030-63453-7
eBook Packages: MedicineMedicine (R0)