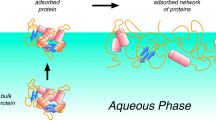
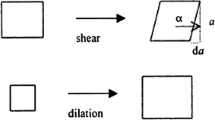
Abstract
Proteins at interfaces present a number of analytical challenges, particularly in the context of biotherapeutic formulations. Proteins typically adsorb to interfaces in thin films or monolayers while being present at high concentrations in the bulk liquid, making sensitivity to behavior directly at the interface an important feature. Additionally, there are multiple timescales of interest, from milliseconds to days, meaning the analytical techniques for probing interfacial behavior need to be adaptable with respect to time resolution. Also, biotherapeutic formulations are usually multicomponent systems, so the techniques need to distinguish between the behavior of the therapeutic protein and other surface-active excipients included to stabilize the protein (e.g., polysorbates and poloxamers). Finally, proteins form amorphous films at interfaces that make traditional interfacial techniques, usually applied to lipids and other well-defined surfactants, less sensitive to structural changes in protein films.
In this chapter, we discuss the strengths and weaknesses of a multitude of interfacial techniques and how these have been applied to understand protein instability at interfaces. First, we examine the multitude of techniques for measuring protein adsorption to interfaces including surface tensiometry, microscopy, and interferometry. Next, we give an overview of rheological tools for evaluating interfacial stresses in protein films. Finally, we discuss a number of spectroscopic techniques for measuring protein structure and conformation within interfacial films. In each section, we review how these various techniques have been employed to understand the dynamics of protein adsorption, unfolding, and aggregation at interfaces and how they could aid in reducing the impact of interfacial stresses during the development of biotherapeutics.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- ATR-FTIR:
-
Attenuated total reflection Fourier transform infrared spectroscopy
- BAM:
-
Brewster angle microscopy
- BLI:
-
Bio-layer interferometry
- DPI:
-
Dual polarization interferometry
- FRAP:
-
Fluorescence recovery after photobleaching
- FRET:
-
Förster resonance energy transfer
- IgG:
-
Immunoglobulin G
- IRRAS:
-
Infrared reflectance absorption spectroscopy
- IRSE:
-
Infrared spectroscopic ellipsometry
- IV:
-
Intravenous
- mAb:
-
Monoclonal antibody
- NR:
-
Neutron reflection
- OWLS:
-
Optical waveguide lightmode spectroscopy
- PM:
-
Polarization modulated
- Q:
-
Wave vector transfer
- QCM-D:
-
Quartz crystal microbalance with dissipation monitoring
- SFG:
-
Sum frequency generation
- SPR:
-
Surface plasmon resonance
- TIRF:
-
Total internal reflection fluorescence
- XRR:
-
X-ray reflectivity
- Δ:
-
Wave phase angle
- Ψ:
-
Wave amplitude difference
References
Buijs J, Lichtenbelt JWT, Norde W, Lyklema J. Adsorption of monoclonal IgGs and their F(ab′)2 fragments onto polymeric surfaces. Colloids Surf B Biointerfaces. 1995;5:11–23. https://doi.org/10.1016/0927-7765(95)98205-2.
Wiseman ME, Frank CW. Antibody adsorption and orientation on hydrophobic surfaces. Langmuir. 2012;28:1765–74. https://doi.org/10.1021/la203095p.
Uchiyama S. Liquid formulation for antibody drugs. Biochim Biophys Acta. 2014;1844:2041–52. https://doi.org/10.1016/j.bbapap.2014.07.016.
Li J, Krause ME, Chen X, et al. Interfacial stress in the development of biologics: fundamental understanding, current practice, and future perspective. AAPS J. 2019;21:44. https://doi.org/10.1208/s12248-019-0312-3.
Sreedhara A, Glover ZK, Piros N, et al. Stability of IgG1 monoclonal antibodies in intravenous infusion bags under clinical in-use conditions. J Pharm Sci. 2012;101:21–30. https://doi.org/10.1002/jps.22739.
Kiese S, Papppenberger A, Friess W, Mahler H-C. Shaken, not stirred: mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97:4347–66. https://doi.org/10.1002/jps.21328.
Biddlecombe JG, Craig AV, Zhang H, et al. Determining antibody stability: creation of solid-liquid interfacial effects within a high shear environment. Biotechnol Prog. 2007;23:1218–22. https://doi.org/10.1021/bp0701261.
Gikanga B, Chen Y, Stauch OB, Maa Y-F. Mixing monoclonal antibody formulations using bottom-mounted mixers: impact of mechanism and design on drug product quality. PDA J Pharm Sci Technol. 2015;69:284–96.
Gerhardt A, Mcgraw NR, Schwartz DK, et al. Protein aggregation and particle formation in prefilled glass syringes. J Pharm Sci. 2014;103:1601–12. https://doi.org/10.1002/jps.23973.
Jones LS, Kaufmann A, Middaugh CR. Silicone oil induced aggregation of proteins. J Pharm Sci. 2005;94:918–27. https://doi.org/10.1002/jps.20321.
Dhar P, Cao Y, Fischer TM, Zasadzinski JA. Active interfacial shear microrheology of aging protein films. Phys Rev Lett. 2010;104:016001. https://doi.org/10.1103/PhysRevLett.104.016001.
Smith C, Li Z, Holman R, et al. Antibody adsorption on the surface of water studied by neutron reflection. mAbs. 2017;9:466–75. https://doi.org/10.1080/19420862.2016.1276141.
Li Z, Li R, Smith C, et al. Neutron reflection study of surface adsorption of Fc, Fab, and the whole mAb. ACS Appl Mater Interfaces. 2017; https://doi.org/10.1021/acsami.7b06131,9,23202.
Kannan A, Shieh IC, Fuller GG. Linking aggregation and interfacial properties in monoclonal antibody-surfactant formulations. J Colloid Interface Sci. 2019;550:128–38. https://doi.org/10.1016/j.jcis.2019.04.060.
Cowsill BJ, Waigh TA, Eapen S, et al. Interfacial structure and history dependent activity of immobilised antibodies in model pregnancy tests. Soft Matter. 2012;8:9847–54. https://doi.org/10.1039/C2SM26133B.
Shieh IC, Patel AR. Predicting the agitation-induced aggregation of monoclonal antibodies using surface tensiometry. Mol Pharm. 2015;12:3184–93. https://doi.org/10.1021/acs.molpharmaceut.5b00089.
Patapoff TW, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear. Pharm Dev Technol. 2009;14:659–64. https://doi.org/10.3109/10837450902911929.
Makievski AV, Fainerman VB, Bree M, et al. Adsorption of proteins at the liquid/air interface. J Phys Chem B. 1998;102:417–25. https://doi.org/10.1021/jp9725036.
Zell ZA, Choi SQ, Leal LG, Squires TM. Microfabricated deflection tensiometers for insoluble surfactants. Appl Phys Lett. 2010;97:133505. https://doi.org/10.1063/1.3491549.
Zell ZA, Isa L, Ilg P, et al. Adsorption energies of poly(ethylene oxide)-based surfactants and nanoparticles on an air–water surface. Langmuir. 2014;30:110–9. https://doi.org/10.1021/la404233a.
Rodahl M, Höök F, Fredriksson C, et al. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997;107:229–46. https://doi.org/10.1039/A703137H.
Voinova MV, Rodahl M, Jonson M, Kasemo B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach. Phys Scr. 1999;59:391. https://doi.org/10.1238/Physica.Regular.059a00391.
Wiseman ME. Antibody adsorption at the silicone oil-water interface: exploring the strengths and limitations of the quartz crystal microbalance technique: Stanford University; 2012.
Li J, Pinnamaneni S, Quan Y, et al. Mechanistic understanding of protein-silicone oil interactions. Pharm Res. 2012;29:1689–97. https://doi.org/10.1007/s11095-012-0696-6.
Dixit N, Maloney KM, Kalonia DS. Protein-silicone oil interactions: comparative effect of nonionic surfactants on the interfacial behavior of a fusion protein. Pharm Res. 2013;30:1848–59. https://doi.org/10.1007/s11095-013-1028-1.
Nirschl M, Reuter F, Vörös J. Review of transducer principles for label-free biomolecular interaction analysis. Biosensors. 2011;1:70–92. https://doi.org/10.3390/bios1030070.
Shen L, Guo A, Zhu X. Tween surfactants: adsorption, self-organization, and protein resistance. Surf Sci. 2011;605:494–9. https://doi.org/10.1016/j.susc.2010.12.005.
Abdiche Y, Malashock D, Pinkerton A, Pons J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the octet. Anal Biochem. 2008;377:209–17.
Ramadan MH, Prata JE, Karácsony O, et al. Reducing protein adsorption with polymer-grafted hyaluronic acid coatings. Langmuir. 2014;30:7485–95. https://doi.org/10.1021/la500918p.
Höök F, Vörös J, Rodahl M, et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf B Biointerfaces. 2002;24:155–70. https://doi.org/10.1016/S0927-7765(01)00236-3.
Cross GH, Reeves AA, Brand S, et al. A new quantitative optical biosensor for protein characterisation. Biosens Bioelectron. 2003;19:383–90. https://doi.org/10.1016/S0956-5663(03)00203-3.
Kim HL, Mcauley A, Livesay B, et al. Modulation of protein adsorption by poloxamer 188 in relation to polysorbates 80 and 20 at solid surfaces. J Pharm Sci. 2014;103:1043–9. https://doi.org/10.1002/jps.23907.
Shieh IC, Waring AJ, Zasadzinski JA. Visualizing the analogy between competitive adsorption and colloid stability to restore lung surfactant function. Biophys J. 2012;102:777–86. https://doi.org/10.1016/j.bpj.2012.01.014.
Leiske DL, Shieh IC, Tse ML. A method to measure protein unfolding at an air–liquid interface. Langmuir. 2016;32:9930–7. https://doi.org/10.1021/acs.langmuir.6b02267.
Walder R, Schwartz DK. Dynamics of protein aggregation at the oil-water interface characterized by single molecule TIRF microscopy. Soft Matter. 2011;7:7616–22. https://doi.org/10.1039/C1SM05232B.
Faulón Marruecos D, Schwartz DK, Kaar JL. Impact of surface interactions on protein conformation. Curr Opin Colloid Interface Sci. 2018;38:45–55. https://doi.org/10.1016/j.cocis.2018.08.002.
Hénon S, Meunier J. Microscope at the Brewster angle: direct observation of first-order phase transitions in monolayers. Rev Sci Instrum. 1991;62:936–9.
Koepf E, Richert M, Braunschweig B, et al. Impact of formulation pH on physicochemical protein characteristics at the liquid-air interface. Int J Pharm. 2018;541:234–45. https://doi.org/10.1016/j.ijpharm.2018.02.009.
Fuller GG, Vermant J. Complex fluid-fluid interfaces: rheology and structure. Annu Rev Chem Biomol Eng. 2012;3:519–43. https://doi.org/10.1146/annurev-chembioeng-061010-114202.
Sorret LL, DeWinter MA, Schwartz DK, Randolph TW. Protein–protein interactions controlling interfacial aggregation of rhIL-1ra are not described by simple colloid models. Protein Sci. n/a:n/a. https://doi.org/10.1002/pro.3382,27,1191.
Lin GL, Pathak JA, Kim DH, et al. Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions. Soft Matter. 2016; https://doi.org/10.1039/C5SM02830B,12,3293.
Choi SQ, Steltenkamp S, Zasadzinski JA, Squires TM. Active microrheology and simultaneous visualization of sheared phospholipid monolayers. Nat Commun. 2011;2:312. https://doi.org/10.1038/ncomms1321.
Kim K, Choi SQ, Zasadzinski JA, Squires TM. Interfacial microrheology of DPPC monolayers at the air-water interface. Soft Matter. 2011;7:7782–9. https://doi.org/10.1039/C1SM05383C.
Frostad JM, Tammaro D, Santollani L, et al. Dynamic fluid-film interferometry as a predictor of bulk foam properties. Soft Matter. 2016;12:9266–79. https://doi.org/10.1039/c6sm01361a.
Kannan A, Shieh IC, Leiske DL, Fuller GG. Monoclonal antibody interfaces: dilatation mechanics and bubble coalescence. Langmuir. 2017;34:630–8. https://doi.org/10.1021/acs.langmuir.7b03790.
Koepf E, Eisele S, Schroeder R, et al. Notorious but not understood: how liquid-air interfacial stress triggers protein aggregation. Int J Pharm. 2018;537:202–12. https://doi.org/10.1016/j.ijpharm.2017.12.043.
Ghazvini S, Kalonia C, Volkin DB, Dhar P. Evaluating the role of the air-solution interface on the mechanism of subvisible particle formation caused by mechanical agitation for an IgG1 mAb. J Pharm Sci. 2016;105:1643–56. https://doi.org/10.1016/j.xphs.2016.02.027.
Goyal DK, Pribil GK, Woollam JA, Subramanian A. Detection of ultrathin biological films using vacuum ultraviolet spectroscopic ellipsometry. Mater Sci Eng B. 2008;149:26–33.
Tiberg F, Joensson B, Tang J, Lindman B. Ellipsometry studies of the self-assembly of nonionic surfactants at the silica-water interface: equilibrium aspects. Langmuir. 1994;10:2294–300.
Nabok A, Tsargorodskaya A, Davis F, Higson SPJ. The study of genomic DNA adsorption and subsequent interactions using total internal reflection ellipsometry. Biosens Bioelectron. 2007;23:377–83.
Kroning A, Furchner A, Aulich D, et al. In situ infrared ellipsometry for protein adsorption studies on ultrathin smart polymer brushes in aqueous environment. ACS Appl Mater Interfaces. 2015;7:12430–9.
Li Z. Interfacial adsorption of monoclonal antibody: a combined study of spectroscopic ellipsometry and neutron reflection: The University of Manchester; 2017.
Campana M, Hosking SL, Petkov JT, et al. Adsorption of bovine serum albumin (BSA) at the oil/water interface: a neutron reflection study. Langmuir. 2015;31:5614–22.
Ruane S, Li Z, Campana M, et al. Interfacial adsorption of a monoclonal antibody and its fab and fc fragments at the oil/water interface. Langmuir. 2019;35:13543.
Xu H, Zhao X, Grant C, et al. Orientation of a monoclonal antibody adsorbed at the solid/solution interface: a combined study using atomic force microscopy and neutron reflectivity. Langmuir. 2006;22:6313–20.
Lavoie H, Desbat B, Vaknin D, Salesse C. Structure of rhodopsin in monolayers at the air− water interface: a PM-IRRAS and X-ray reflectivity study. Biochemistry. 2002;41:13424–34.
Evers F, Shokuie K, Paulus M, et al. Characterizing the structure of protein layers adsorbed onto functionalized surfaces by means of in-situ X-ray reflectivity. Eur Phys J Spec Top. 2009;167:185–9. https://doi.org/10.1140/epjst/e2009-00956-1.
Richter AG, Kuzmenko I. Using in situ X-ray reflectivity to study protein adsorption on hydrophilic and hydrophobic surfaces: benefits and limitations. Langmuir. 2013;29:5167–80.
Wang J, Paszti Z, Even MA, Chen Z. Interpretation of sum frequency generation vibrational spectra of interfacial proteins by the thin film model. J Phys Chem B. 2004;108:3625–32.
Chen X, Wang J, Sniadecki JJ, et al. Probing α-helical and β-sheet structures of peptides at solid/liquid interfaces with SFG. Langmuir. 2005;21:2662–4.
Weidner T, Castner DG. SFG analysis of surface bound proteins: a route towards structure determination. Phys Chem Phys. 2013;15:12516–24.
Yan ECY, Wang Z, Fu L. Proteins at interfaces probed by chiral vibrational sum frequency generation spectroscopy. J Phys Chem B. 2015;119:2769–85.
Fu L, Ma G, Yan ECY. In situ misfolding of human islet amyloid polypeptide at interfaces probed by vibrational sum frequency generation. J Am Chem Soc. 2010;132:5405–12.
Fu L, Liu J, Yan ECY. Chiral sum frequency generation spectroscopy for characterizing protein secondary structures at interfaces. J Am Chem Soc. 2011;133:8094–7.
Fu L, Wang Z, Yan ECY. Chiral vibrational structures of proteins at interfaces probed by sum frequency generation spectroscopy. Int J Mol Sci. 2011;12:9404–25.
Fu L, Wang Z, Yan ECY. N-H stretching modes around 3300 wavenumber from peptide backbones observed by chiral sum frequency generation vibrational spectroscopy. Chirality. 2014;26:521–4.
Sackett DL, Wolff J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal Biochem. 1987;167:228–34.
Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25:1487–99. https://doi.org/10.1007/s11095-007-9516-9.
Dluhy RA, Cornell DG. In situ measurement of the infrared spectra of insoluble monolayers at the air-water interface. J Phys Chem. 1985;89:3195–7.
Meinders MB, van den Bosch GG, de Jongh HH. Adsorption properties of proteins at and near the air/water interface from IRRAS spectra of protein solutions. Eur Biophys J. 2001;30:256–67.
Meinders MBJ, De Jongh HHJ. Limited conformational change of β-lactoglobulin when adsorbed at the air–water interface. Biopolym Orig Res Biomol. 2002;67:319–22.
Martin AH, Meinders MBJ, Bos MA, et al. Conformational aspects of proteins at the air/water interface studied by infrared reflection− absorption spectroscopy. Langmuir. 2003;19:2922–8.
Desroches MJ, Chaudhary N, Omanovic S. PM-IRRAS investigation of the interaction of serum albumin and fibrinogen with a biomedical-grade stainless steel 316LVM surface. Biomacromolecules. 2007;8:2836–44.
Chittur KK. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials. 1998;19:357–69.
Déjardin P. Proteins at solid-liquid interfaces: Springer; 2006.
Sethuraman A, Vedantham G, Imoto T, et al. Protein unfolding at interfaces: slow dynamics of α-helix to β-sheet transition. Proteins Struct Funct Bioinforma. 2004;56:669–78.
Mudunkotuwa IA, Al Minshid A, Grassian VH. ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid–solid interface in environmentally and biologically relevant media. Analyst. 2014;139:870–81.
Kim J, Somorjai GA. Molecular packing of lysozyme, fibrinogen, and bovine serum albumin on hydrophilic and hydrophobic surfaces studied by infrared−visible sum frequency generation and fluorescence microscopy. J Am Chem Soc. 2003;125:3150–8. https://doi.org/10.1021/ja028987n.
Liao Z, Lampe JW, Ayyaswamy PS, et al. Protein assembly at the air-water interface studied by fluorescence microscopy. Langmuir. 2011;27:12775–81. https://doi.org/10.1021/la203053g.
Cullen DC, Lowe CR. AFM studies of protein adsorption: 1 Time-resolved protein adsorption to highly oriented pyrolytic graphite. J Colloid Interface Sci. 1994;166:102–8.
Hlady V, Buijs J. Protein adsorption on solid surfaces. Curr Opin Biotechnol. 1996;7:72–7.
Browne MM, Lubarsky GV, Davidson MR, Bradley RH. Protein adsorption onto polystyrene surfaces studied by XPS and AFM. Surf Sci. 2004;553:155–67.
Mackie AR, Gunning AP, Wilde PJ, Morris VJ. Orogenic displacement of protein from the air/water interface by competitive adsorption. J Colloid Interface Sci. 1999;210:157–66.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Shieh, I.C., Cheng, Y. (2021). Analytical Techniques for Evaluating Protein Instability at Interfaces. In: Li, J., Krause, M.E., Tu, R. (eds) Protein Instability at Interfaces During Drug Product Development. AAPS Advances in the Pharmaceutical Sciences Series, vol 43. Springer, Cham. https://doi.org/10.1007/978-3-030-57177-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-57177-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57176-4
Online ISBN: 978-3-030-57177-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)