Abstract
The growing concerns on the environment in recent years have influenced the usage of renewable sources as alternative materials to create a platform for the development of new technology with possible economic potential. Adsorbents derived from agricultural wastes have hidden economic values which could be benefited by transforming the agricultural wastes into valuable and useful products. Numerous agricultural wastes such as skins/peels, cores, pits, leaves, brunches, and pericarp are being produced in plantation and processing industries. The agricultural wastes have exhibited the potential usage as an adsorbent to remove contaminants from water environment which conserve the natural environment and resources mainly in the ecology system sustainably, for the reason that this utilization converts the agricultural wastes into value-added product and at the same time decontaminate polluted water source. This application on the utilization of agricultural wastes is not only good for a sustainable environment but is also suitable for rural economic development, meaning possible increases in profit for farmers and the agricultural industry. This chapter provides insight on some findings on heavy metal removal by adsorbents produced from agricultural wastes. The chapter also discusses the situation of the heavy metals in the environment, parameters affecting the adsorption process of the heavy metals, kinetic models, and adsorption isotherms that are associated with the agricultural waste-derived adsorbents. The development of the adsorbents from agricultural waste biomass and the prospect of developing hybrid adsorbent and magnetic adsorbent have attracted many researchers worldwide in performing research work on the application to water and wastewater treatment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- α :
-
Initial adsorption rate in mg/g·min unit
- β :
-
Desorption constant in g/mg unit
- ɛ :
-
Polanyi potential
- Al:
-
Aluminium
- As:
-
Arsenic
- As(III):
-
Arsenite
- As(V):
-
Arsenate
- B(II):
-
Boron(II) cation
- Cd:
-
Cadmium
- Cd(II), Cd2+:
-
Cadmium(II) cation
- Cd(OH)+:
-
Cadmium hydroxide ion in its +1 oxidation state
- C 0 :
-
Initial concentration of the solution in mg/L unit
- C c :
-
Equilibrium concentration in mg/L unit
- C i :
-
Initial concentration in mg/L unit
- Co:
-
Cobalt
- Co(II):
-
Cobalt (II) ion
- Co-Fe-MBC:
-
Cobalt-iron-magnetic biochar
- Co(NO3)2:
-
Cobaltous nitrate
- Cu:
-
Copper
- Cu(II) :
-
Copper (II) cation
- Cr:
-
Chromium
- Cr(III):
-
Chromium (III)
- Cr(VI):
-
Hexavalent chromium
- CrO42−:
-
Chromium oxoanion
- Cr2O72−:
-
Dichromate ion
- Cs(I):
-
Caesium(I) ion
- DALYs:
-
Disability-Adjusted Life Years
- E :
-
Mean free energy in (kJ/mol) unit
- Fe:
-
Iron
- Fe(III):
-
Element iron in its +3 oxidation state
- FeCl3:
-
Iron(III) chloride
- FeCl3.6H2O:
-
Iron(III) chloride hexahydrate
- Fe-MBC:
-
Iron-magnetic biochar
- H+:
-
A cationic form of atomic hydrogen
- Hg:
-
Mercury
- Hg(II):
-
Mercury(II) cation
- HCrO4−:
-
Hydrogen chromate ion
- k 1 :
-
Rate constant in min−1 unit
- k 2 :
-
Rate constant in g/mg·min unit
- K F :
-
Freundlich isotherm constant in (mg1–1/nL1/ng−1) unit
- K L :
-
Langmuir isotherm constant in L/mg unit
- K DR :
-
Dubinin-Radushkevich isotherm constant in (mol2/kJ2) unit
- MnFe2O4:
-
Manganese ferrite
- MnSO4.H2O:
-
Manganese sulphate monohydrate
- n :
-
Adsorption intensity
- 1/n:
-
Linearity degree of the relationship between the solution concentration and adsorption capacity
- Ni:
-
Nickel
- Ni(II):
-
Nickel (II) ion
- NiCl2:
-
Nickel(II) chloride
- OH−:
-
Hydroxide, a diatomic anion
- Pb:
-
Lead
- Pb(II):
-
Lead(II) ion
- qe, Qe:
-
Equilibrium adsorption capacity in mg/g unit
- Q m :
-
Maximum adsorption capacity in mg/g unit
- q t :
-
Adsorption capacity at time t in mg/g unit
- R:
-
Universal gas constant (8.314 J/mol·K)
- Se(IV):
-
Selenium(IV) ion
- t :
-
Time in min unit
- T :
-
Temperature in K unit
- U(VI):
-
Uranyl ion
- V(V):
-
Vanadium(V) ion
- WHO:
-
World Health Organization
- Zn:
-
Zinc
- Zn(II):
-
Zinc ion
References
Pure Earth, Green Cross Switzerland (2016) The world's worst pollution problems 2016: toxics beneath our feet. https://www.worstpolluted.org/docs/WorldsWorst2016.pdf
Fergusson JE (1990) The heavy elements: chemistry, environmental impact and health effects. Pergamon Press, Oxford
Green Cross Switzerland, Blacksmith Institute (2012) The world's worst pollution problems: assessing health risks at hazardous waste sites. https://www.worstpolluted.org/files/FileUpload/files/WWPP_2012.pdf
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. https://doi.org/10.1016/j.tox.2011.03.001
National Toxicology Program (2016) Report on carcinogens, 14th edn. U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology, Experientia supplementum, vol 101. Springer, Basel. https://doi.org/10.1007/978-3-7643-8340-4_6
Solomon F (2008) Impacts of metals on aquatic ecosystems and human health. Environ Commun:14–19
Wright DA, Welbourn P (2002) Environmental toxicology. Cambridge University Press, Cambridge, UK
Bradl H (2005) Heavy metals in the environment: origin, interaction and remediation. Elsevier/Academic Press, London
Wang Z, Rossman TG (1996) The carcinogenecity of arsenic. In: Chang T (ed) Toxicology of metals. CRC Press, Boca Raton, pp 221–229
Tchounwou PB, Wilson BA, Abdelgnani AA, Ishaque AB, Patlolla AK (2002) Differential cytotoxicity and gene expression in human liver carcinoma (Hepg2) cells exposed to arsenic trioxide and monosodium acid methanearsonate (Msma). Int J Mol Sci 3:1117–1132
Waalkes MP, Liu J, Ward JM, Diwan LA (2004) Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology 198:31–38
Flora SJS, Pachauri V (2010) Chelation in metal Intoxification. Int J Environ Res Public Health 7:2745–2788
Rzymski P, Rzymski P, Tomcyzk K, Niedzielki P, Jakubowski K, Poniezialek B, Opala T (2014) Metal status in human endometrium: relation to cigarette smoking and histological lesions. Environ Res 132:328–333
Dayan AD, Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenecity and allergenicity; review of the literature from 1985 to 2000. Hum Exp Toxicol 20(9):439–451
Gal J, Hursthouse A, Tatner P, Stewart F, Welton R (2008) Cobalt and secondary poisoning in terrestrial food chain: data review and research gaps to support risk assessment. Environ Int 34:821–838
Burkitt MJ (2001) A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, alpha-tocopherol, thiols, and ceruloplasmin. Arch Biochem Biophys 394:117–135
Lee DH, Liu DY, Jacobs DR Jr, Shin HR, Song K, Lee IK, Hider RC (2006) Common presence of non-transferrin-bound Iron among patients with type 2 diabetes. Diabetes Care 29:1090–1095
Touyz RM, Schiffrin EL (2004) Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122:339–352
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Inoue M, Sato EF, Nishikawa M, Park AM, Kari Y, Imada I, Utsumi K (2003) Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem 10:2495–2505
Borja-Aburto V, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J (1999) Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol 150(6):590–597
Szkup-Jablonska M, Karakiewicz B, Grochans E, Jurczak A, Nowak-Starz G, Rotter I, Prokopowicz A (2012) Effects of blood lead and cadmium levels on the functioning of children with behaviour disorders in the family environment. Ann Agric Environ Med 19(2):241–246
Kaul B, Sandhu RS, Depratt C, Reyes F (1999) Follow-up screening of lead-poisoned children near an auto battery recycling plant, Haina, Dominican Republic. Environ Health Perspect 107(11):917–720
Hertz-Picciotto I (2000) The evidence that lead increases the risk for spontaneous abortion. Am J Ind Med 38:300–309
Wasana HMS, Perera GDRK, Gunawardena PDS, Fernando PS, Bandara J (2017) Who water quality standards vs synergic effect(S) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci Rep 7:42516. https://doi.org/10.1038/srep42516
Lim HS, Lee HH, Kim TH, Lee BR (2016) Relationship between heavy metal exposure and bone mineral density in Korean adult. J Bone Metabol 23(4):223–231. https://doi.org/10.11005/jbm.2016.23.4.223
Corradi M, Mutti A (2011) Metal ions affecting the pulmonary and cardiovasular system. Metal Ions Life Sci 8:81–105
Gautam RK, Mudhoo A, Lofrano G, Chattopadhyaya MC (2014) Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J Environ Chem Eng 2(1):239–259. https://doi.org/10.1016/j.jece.2013.12.019
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY et al (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresource Technol 148:574–585. https://doi.org/10.1016/j.biortech.2013.08.124
Ghani WWAK, Abdullah MF, Loung C, Ho C, Matori K (2008) Characterization of vitrified malaysian agrowaste ashes as potential recycling material. Int J Eng Technol 5:111–117
Mo J, Yang Q, Zhang N, Zhang W, Zheng Y, Zhang Z (2018) A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J Environ Manag 227:395–405
Georgieva VG, Gonsalvesh L, Tavlieva MP (2020) Thermodynamics and kinetics of the removal of nickel (II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells. J Mol Liq 312:112788
Khattak MMUR, Zahoor M, Muhammad B, Khan FA, Ullah R, AbdEI-Salam NM (2017) Removal of heavy metals from drinking water by magnetic carbon nanostructures prepared from biomass. J Nanomater 2017:5670371, 10 pages
Thines KR, Abdullah EC, Mubarak NM, Ruthiraan M (2017) Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: a review. Renew Sust Energ Rev 67:257–276
Yi Y, Huang Z, Lu B, Xian J, Tsang PE, Cheng W, Fang J, Fang Z (2020) Magnetic biochar for environmental remediation: a review. Bioresour Technol 298:122468
Kılıç M, Kırbıyık Ç, Çepelioğullar Ö, Pütün AE (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surface Sci 283:856–862. https://doi.org/10.1016/j.apsusc.2013.07.033
Mahajan G, Sud D (2013) Application of Ligno-cellulosic waste material for heavy metal ions removal from aqueous solution. J Environ Chem Eng 1(4):1020–1027. https://doi.org/10.1016/j.jece.2013.08.013
Shang J, Pi J, Zong M, Wang Y, Li W, Liao Q (2016) Chromium removal using magnetic biochar derived from herb-residue. J Taiwan Ins Chem Eng 68:289–294. https://doi.org/10.1016/j.jtice.2016.09.012
Wang W, Wang X, Wang X, Yang L, Wu Z, Xia S, Zhao J (2013) Cr(vi) removal from aqueous solution with bamboo charcoal chemically modified by iron and cobalt with the assistance of microwave. J Environ Sci 25(9):1726–1735. https://doi.org/10.1016/S1001-0742(12)60247-2
Gupta VK, Pathania D, Sharma S, Singh P (2013) Preparation of bio-based porous carbon by microwave assisted phosphoric acid activation and its use for adsorption of Cr(vi). J Coll Interface Sci 401:125–132. https://doi.org/10.1016/j.jcis.2013.03.020
Khan Rao RA, Ikram S, Uddin MK (2014) Removal of cd(ii) from aqueous solution by exploring the biosorption characteristics of Gaozaban (Onosma Bracteatum). J Environ Chem Eng 2(2):1155–1164. https://doi.org/10.1016/j.jece.2014.04.008
Tounsadi H, Khalidi A, Machrouhi A, Farnane M, Elmoubarki R, Elhalil A et al (2016) Highly efficient activated carbon from Glebionis Coronaria L. biomass: optimization of preparation conditions and heavy metals removal using experimental design approach. J Environ Chem Eng 4(4, Part A):4549–4564. https://doi.org/10.1016/j.jece.2016.10.020
Wang H, Gao B, Wang S, Fang J, Xue Y, Yang K (2015) Removal of Pb(Ii), Cu(Ii), and Cd(Ii) from aqueous solutions by biochar derived from Kmno4 treated Hickory wood. Bioresource Technol 197:356–362. https://doi.org/10.1016/j.biortech.2015.08.132
Shrestha S, Son G, Lee SH, Lee TG (2013) Isotherm and thermodynamic studies of Zn (Ii) adsorption on lignite and coconut shell-based activated carbon fiber. Chemosphere 92(8):1053–1061. https://doi.org/10.1016/j.chemosphere.2013.02.068
Bediako JK, Reddy DHK, Song MH, Wei W, Lin S, Yun YS (2017) Preparation, characterization and lead adsorption study of tripolyphosphate-modified waste lyocell fibers. J Environ Chem Eng 5(1):412–421. https://doi.org/10.1016/j.jece.2016.12.022
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2013) Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J Environ Chem Eng 1(3):589–599. https://doi.org/10.1016/j.jece.2013.06.028
Hodaifa G, Ochando-Pulido JM, Driss Alami SB, Rodriguez-Vives S, Martinez-Ferez A (2013) Kinetic and thermodynamic parameters of Iron adsorption onto olive stones. Indus Crops Prod 49:526–534. https://doi.org/10.1016/j.indcrop.2013.05.039
Kushwaha S, Sudhakar PP (2013) Sorption of uranium from aqueous solutions using palm-Shell-based adsorbents: a kinetic and equilibrium study. J Environ Radioactiv 126:115–124. https://doi.org/10.1016/j.jenvrad.2013.07.021
Wang C, Gu L, Liu X, Zhang X, Cao L, Hu X (2016) Sorption behavior of Cr(Vi) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int Biodeteriorat Biodegrad 111:78–84. https://doi.org/10.1016/j.ibiod.2016.04.029
Verma A, Kumar S, Kumar S (2016) Biosorption of lead ions from the aqueous solution by Sargassum Filipendula: equilibrium and kinetic studies. J Environ Chem Eng 4(4, Part A):4587–4599. https://doi.org/10.1016/j.jece.2016.10.026
Kaya K, Pehlivan E, Schmidt C, Bahadir M (2014) Use of modified wheat bran for the removal of chromium(Vi) from aqueous solutions. Food Chem 158:112–117. https://doi.org/10.1016/j.foodchem.2014.02.107
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99:6709–6724
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15(1):86–102. https://doi.org/10.1007/s12257-009-0199-4
Zhong QQ, Yue QY, Gao BY, Li Q, Xu X (2013) A novel amphoteric adsorbent derived from biomass materials: synthesis and adsorption for Cu(Ii)/Cr(Vi) in single and binary systems. Chem Eng J 229:90–98. https://doi.org/10.1016/j.cej.2013.05.083
Dong YB, Lin H (2015) Adsorption of Cu2+ from aqueous solution by modified biomass material. Trans Nonferrous Met Soc China 25(3):991–996. https://doi.org/10.1016/S1003-6326(15)63689-5
World Health Organization (2006) Guidelines for drinking water quality [electronic resource]: incorporating first addendum, 3rd edn, vol 1, Recommendations. World Health Organization
Ullah I, Nadeem R, Iqbal M, Manzoor Q (2013) Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecol Eng 60:99–107. https://doi.org/10.1016/j.ecoleng.2013.07.028
Ghasemi M, Naushad M, Ghasemi N, Khosravi-fard Y (2014) A novel agricultural waste based adsorbent for the removal of Pb(Ii) from aqueous solution: kinetics, equilibrium and thermodynamic studies. J Indus Eng Chem 20(2):454–461. https://doi.org/10.1016/j.jiec.2013.05.002
Gupta A, Vidyarthi SR, Sankararamakrishnan N (2015) Concurrent removal of as(Iii) and as(V) using green low cost functionalized biosorbent – Saccharum Officinarum bagasse. J Environ Chem Eng 3(1):113–121. https://doi.org/10.1016/j.jece.2014.11.023
Martins AE, Pereira MS, Jorgetto AO, Martines MAU, Silva RIV, Saeki MJ, Castro GR (2013) The reactive surface of Castor leaf [Ricinus Communis L.] powder as a green adsorbent for the removal of heavy metals from natural river water. Appl Surface Sci 276:24–30. https://doi.org/10.1016/j.apsusc.2013.02.096
Pillai SS, Deepa B, Abraham E, Girija N, Geetha P, Jacob L, Koshy M (2013) Biosorption of Cd(Ii) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicol Environ Safety 98:352–360. https://doi.org/10.1016/j.ecoenv.2013.09.003
Ben-Ali S, Jaouali I, Souissi-Najar S, Ouederni A (2017) Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Cleaner Product 142(Part 4):3809–3821. https://doi.org/10.1016/j.jclepro.2016.10.081
Yargıç AŞ, Yarbay Şahin RZ, Özbay N, Önal E (2015) Assessment of toxic copper(Ii) biosorption from aqueous solution by chemically-treated tomato waste. J Cleaner Product 88:152–159. https://doi.org/10.1016/j.jclepro.2014.05.087
López-García M, Lodeiro P, Herrero R, Barriada JL, Rey-Castro C, David C, Sastre de Vicente ME (2013) Experimental evidences for a new model in the description of the adsorption-coupled reduction of Cr(Vi) by protonated banana skin. Bioresource Technol 139:181–189. https://doi.org/10.1016/j.biortech.2013.04.044
Taşar Ş, Kaya F, Özer A (2014) Biosorption of lead(Ii) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng 2(2):1018–1026. https://doi.org/10.1016/j.jece.2014.03.015
Albadarin AB, Glocheux Y, Ahmad MNM, Walker GM, Mangwandi C (2014) Novel comparison of kinetic models for the adsorption-coupled reduction of Cr(Vi) using untreated date pit biomaterial. Ecol Eng 70:200–205. https://doi.org/10.1016/j.ecoleng.2014.05.002
Chang J, Ma J, Ma Q, Zhang D, Qiao N, Hu M, Ma H (2016) Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl Clay Sci 119(Part 1):132–140. https://doi.org/10.1016/j.clay.2015.06.038
Qiu H, Lv L, Pan BC, Zhang QJ, Zhang WM, Zhang QX (2009) Critical review in adsorption kinetic models. J Zheijang Univ Sci A 10(5):716–724. https://doi.org/10.1631/jzus.A0820524
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Proc Safety Environ Protect 76(2):183–191. https://doi.org/10.1205/095758298529326
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124. https://doi.org/10.1016/S0923-0467(98)00076-1
Ho YSR (2006) Review of second-order models for adsorption systems. J Hazard Mater 136(3):681–689. https://doi.org/10.1016/j.jhazmat.2005.12.043
Foo KY, Lee LK, Hameed BH (2013) Preparation of banana frond activated carbon by microwave-induced activation for the removal of boron and total iron from landfill leachate. Chem Eng J 223:604–610
Tabaraki R, Nateghi A, Asbchin SA (2014) Biosorption of lead (II) ions on Sargassum ilicifolium: application of response surface methodology. Int Biodeterior Biodegradation 93:145–152. https://doi.org/10.1016/j.ibiod.2014.03.022
Hu H, Zhao Q (2018) Optimization extraction and functional properties of soluble dietary fiber from pineapple pomace obtained by shear homogenization-assisted extraction. RSC Adv 8:41117–41130. https://doi.org/10.1039/C8RA06928J
Gulipalli CHS, Prasad B, Wasewar KL (2011) Batch study, Equilibrium and kinetics of adsorption of selenium using rice husk ash (RHA). J Eng Sci Technol 6(5):590–609
Gurung M, Adhikari BB, Alam S, Kawakita H, Ohto K, Inoue K, Harada H (2013) Adsorptive removal of Cs(I) from aqueous solution using polyphenols enriched biomass-based adsorbents. Chem Eng J 231:113–120. https://doi.org/10.1016/j.cej.2013.06.028
Rangabhashiyam S, Anu N, Giri Nandagopal MS, Selvaraju N (2014) Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J Environ Chem Eng 2(1):398–414
Anirudhan TS, Chandran R (2015) Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): kinetic and competitive adsorption isotherm. Process Saf Environ Prot 95:215–225
Mahamad MN, Zaini MAA, Zakaria ZA (2015) Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int Biodeteriorat Biodegrad 102:274–280. https://doi.org/10.1016/j.ibiod.2015.03.009
Rocha CG, Zaia DAM, Alfaya RVDS, Alfaya AADS (2009) Use of rice straw as biosorbent for removal of Cu(II), Zn(II), Cd(II) and Hg(II) ions in industrial effluents. J Hazard Mater 166(1):383–388. https://doi.org/10.1016/j.jhazmat.2008.11.074
Yakout SM, Metwally SS, El-Zakla T (2013) Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids. Appl Surf Sci 280:745–750. https://doi.org/10.1016/j.apsusc.2013.05.055
Li M, Liu Q, Guo L, Zhang Y, Lou Z, Wang Y, Qian G (2013) Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresource Technol 141:83–88. https://doi.org/10.1016/j.biortech.2012.12.096
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
Glossary
- Adsorbent :
-
A material which will allow a liquid, gas, or dissolved solid to adhere to its surface.
- Adsorption :
-
A process by which a solid holds molecules of a gas or liquid or solute as a thin film.
- Adsorption capacity :
-
The amount of adsorbate taken up by the adsorbent per unit mass (or volume) of the adsorbent.
- Agricultural wastes :
-
Unwanted or unsalable materials produced wholly from agricultural operations directly related to the growing of crops or raising of animals for the primary purpose of making a profit or for a livelihood.
- Anionic :
-
A negatively charged ion, as one attracted to the anode in electrolysis. Any negatively charged atom or group of atoms.
- Aquatic ecosystem :
-
A group of interacting organisms which are dependent on one another and their water environment for nutrients and shelter.
- Biochar :
-
A charcoal-like substance that is made by burning organic material from agricultural and forestry wastes (also called biomass) in a controlled process called pyrolysis.
- Biomass :
-
Biomass is organic material that comes from plants and animals, and it is a renewable source of energy. Biomass contains stored energy from the sun.
- Cationic :
-
A positively charged ion that is attracted to the cathode in electrolysis. Any positively charged atom or group of atoms.
- Cellulose :
-
A natural linear polymer (polysaccharide) with a molecular repeat unit comprised of a pair of d-anhydroglucose ring units joined by β-1 → 4 glycosidic oxygen linkages around which the molecular chain can bend and twist.
- Chemical precipitation :
-
Formation of a separable solid substance from a solution, either by converting the substance into an insoluble form or by changing the composition of the solvent to diminish the solubility of the substance in it.
- Coagulation :
-
A process for combining small particles into larger aggregates (flocs). A chemical water treatment technique typically applied prior to sedimentation and filtration.
- Carbonization :
-
A process by which solid residues with increasing content of the element carbon are formed from organic material usually by pyrolysis in an inert atmosphere.
- Ecosystem :
-
A geographic area where plants, animals, and other organisms, as well as weather and landscape, work together to form a bubble of life.
- Electrochemical treatment :
-
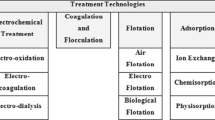
Uses electrolysis and fuel cell technology to treat water and wastewater such as electrocoagulation/ electro-flotation, electrodialysis, electro-reduction, sono-electrolysis, and electro-oxidation.
- Filtration :
-
A process of separating suspended solid matter from a liquid, by causing the latter to pass through the pores of some substance (filter).
- Heavy metals :
-
Metallic cation and anion that have relatively high density and toxic or poisonous at low concentrations.
- Hemicellulose :
-
The second most abundant chemical constituent of woody and grassy biomass and is present along with cellulose in almost all terrestrial plant cell walls. Also known as polyose.
- Human health :
-
A complete state of physical, psychological, and social wellbeing of individuals.
- Ion exchange :
-
A reversible chemical reaction where dissolved ions are removed from the solution and replaced with other ions of the same or similar electrical charge.
- Kinetics :
-
The study of reaction rates. The branch of mechanics that deals with the actions of forces in producing or changing the motion of masses.
- Lignin :
-
An irregular polyphenolic polymer which is synthesized by dehydrogenative polymerization of phenyl propanoid units, namely, coniferyl alcohol, sinapyl alcohol, and coumaryl alcohol, which corresponds to guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) structures of lignin, respectively.
- Physicochemical :
-
Pertaining both physical and chemical properties, changes, and reactions of or according to physical chemistry.
- Selectivity :
-
The discrimination demonstrated by a reagent in competitive attack on two or more substrates or on two or more positions in the same substrate. It is quantitatively formulated by ratios of rate constants of the competing reactions or by the logarithms of these ratios.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lim, SF., Karim, S.K.A., Chua, S.N.D., Lim, BH. (2021). Agricultural Waste-Derived Adsorbents for Decontamination of Heavy Metals. In: Wang, L.K., Wang, MH.S., Hung, YT., Shammas, N.K. (eds) Integrated Natural Resources Management. Handbook of Environmental Engineering, vol 20. Springer, Cham. https://doi.org/10.1007/978-3-030-55172-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-55172-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55171-1
Online ISBN: 978-3-030-55172-8
eBook Packages: EngineeringEngineering (R0)