Abstract
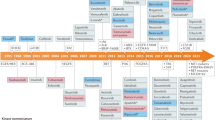
Research on kinase-targeting drugs has made great strides over the last 30 years and is attracting greater attention for the treatment of yet more kinase-related diseases. Currently, 42 kinase drugs have been approved by the FDA, most of which (Wilson et al., Cancer Research 78(1):15–29, 2018) are Type I/II inhibitors. Notwithstanding these advances, it is desirable to target additional kinases for drug development as more than 200 diseases, particularly cancers, are directly associated with aberrant kinase regulation and signaling. Here, we review the extant Type I/II drugs systematically to obtain insights into the binding pocket characteristics, the associated features of Type I/II drugs, and the mechanism of action to facilitate future kinase drug design and discovery. We conclude by summarizing the main successes and limitations of targeting kinases for the development of drugs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Adams, J. A. (2001). Kinetic and catalytic mechanisms of protein kinases. Chemical Reviews, 101(8), 2271–2290.
Logue, J. S., & Morrison, D. K. (2012). Complexity in the signaling network: Insights from the use of targeted inhibitors in cancer therapy. Genes and Development, 26(7), 641–650. https://doi.org/10.1101/gad.186965.112.
Manning, G., Whyte, D. B., Martinez, R., Hunter, T., & Sudarsanam, S. (2002). The protein kinase complement of the human genome. Science, 298(5600), 1912–1934. https://doi.org/10.1126/science.1075762.
Cell Signaling Technology. Kinase-disease associations. CST. https://www.cellsignal.com/contents/resources-reference-tables/kinase-disease-associations/science-tables-kinase-disease.
Lahiry, P., Torkamani, A., Schork, N. J., & Hegele, R. A. (2010). Kinase mutations in human disease: Interpreting genotype-phenotype relationships. Nature Reviews Genetics, 11(1), 60–74. https://doi.org/10.1038/nrg2707.
Gharwan, H., & Groninger, H. (2016). Kinase inhibitors and monoclonal antibodies in oncology: Clinical implications. Nature Reviews Clinical Oncology, 13(4), 209–227. https://doi.org/10.1038/nrclinonc.2015.213.
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., Jr., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558. https://doi.org/10.1126/science.1235122.
Wilson, L. J., Linley, A., Hammond, D. E., Hood, F. E., Coulson, J. M., MacEwan, D. J., Ross, S. J., Slupsky, J. R., Smith, P. D., Eyers, P. A., & Prior, I. A. (2018). New perspectives, opportunities, and challenges in exploring the human protein kinome. Cancer Research, 78(1), 15–29. https://doi.org/10.1158/0008-5472.CAN-17-2291.
Zhang, J., Yang, P. L., & Gray, N. S. (2009). Targeting cancer with small molecule kinase inhibitors. Nature Reviews Cancer, 9(1), 28–39. https://doi.org/10.1038/nrc2559.
U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
Knapp, S. (2018). New opportunities for kinase drug repurposing and target discovery. British Journal of Cancer, 118(7), 936–937. https://doi.org/10.1038/s41416-018-0045-6.
Muller, S., Chaikuad, A., Gray, N. S., & Knapp, S. (2015). The ins and outs of selective kinase inhibitor development. Nature Chemical Biology, 11(11), 818–821. https://doi.org/10.1038/nchembio.1938.
Ferguson, F. M., & Gray, N. S. (2018). Kinase inhibitors: The road ahead. Nature Reviews. Drug Discovery, 17(5), 353–377. https://doi.org/10.1038/nrd.2018.21.
Klaeger, S., Heinzlmeir, S., Wilhelm, M., Polzer, H., Vick, B., Koenig, P. A., Reinecke, M., Ruprecht, B., Petzoldt, S., Meng, C., Zecha, J., Reiter, K., Qiao, H., Helm, D., Koch, H., Schoof, M., Canevari, G., Casale, E., Depaolini, S. R., Feuchtinger, A., Wu, Z., Schmidt, T., Rueckert, L., Becker, W., Huenges, J., Garz, A. K., Gohlke, B. O., Zolg, D. P., Kayser, G., Vooder, T., Preissner, R., Hahne, H., Tonisson, N., Kramer, K., Gotze, K., Bassermann, F., Schlegl, J., Ehrlich, H. C., Aiche, S., Walch, A., Greif, P. A., Schneider, S., Felder, E. R., Ruland, J., Medard, G., Jeremias, I., Spiekermann, K., & Kuster, B. (2017). The target landscape of clinical kinase drugs. Science, 358(6367), eaan4368. https://doi.org/10.1126/science.aan4368.
Wu, P., Nielsen, T. E., & Clausen, M. H. (2015). FDA-approved small-molecule kinase inhibitors. Trends in Pharmacological Sciences, 36(7), 422–439. https://doi.org/10.1016/j.tips.2015.04.005.
Zhao, Z., Xie, L., Xie, L., & Bourne, P. E. (2016). Delineation of polypharmacology across the human structural kinome using a functional site interaction fingerprint approach. Journal of Medicinal Chemistry, 59(9), 4326–4341. https://doi.org/10.1021/acs.jmedchem.5b02041.
Wu, P., Clausen, M. H., & Nielsen, T. E. (2015). Allosteric small-molecule kinase inhibitors. Pharmacology and Therapeutics, 156, 59–68. https://doi.org/10.1016/j.pharmthera.2015.10.002.
Rice, K. D., Aay, N., Anand, N. K., Blazey, C. M., Bowles, O. J., Bussenius, J., Costanzo, S., Curtis, J. K., Defina, S. C., Dubenko, L., Engst, S., Joshi, A. A., Kennedy, A. R., Kim, A. I., Koltun, E. S., Lougheed, J. C., Manalo, J. C., Martini, J. F., Nuss, J. M., Peto, C. J., Tsang, T. H., Yu, P., & Johnston, S. (2012). Novel carboxamide-based allosteric MEK inhibitors: Discovery and optimization efforts toward XL518 (GDC-0973). ACS Medicinal Chemistry Letters, 3(5), 416–421. https://doi.org/10.1021/ml300049d.
Zhang, J., Adrián, F. J., Jahnke, W., Cowan-Jacob, S. W., Li, A. G., Iacob, R. E., Sim, T., Powers, J., Dierks, C., Sun, F., Guo, G.-R., Ding, Q., Okram, B., Choi, Y., Wojciechowski, A., Deng, X., Liu, G., Fendrich, G., Strauss, A., Vajpai, N., Grzesiek, S., Tuntland, T., Liu, Y., Bursulaya, B., Azam, M., Manley, P. W., Engen, J. R., Daley, G. Q., Warmuth, M., & Gray, N. S. (2010). Targeting Bcr–Abl by combining allosteric with ATP-binding-site inhibitors. Nature, 463(7280), 501–506. https://doi.org/10.1038/nature08675.
Hirai, H., Sootome, H., Nakatsuru, Y., Miyama, K., Taguchi, S., Tsujioka, K., Ueno, Y., Hatch, H., Majumder, P. K., Pan, B. S., & Kotani, H. (2010). MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular Cancer Therapeutics, 9(7), 1956–1967. https://doi.org/10.1158/1535-7163.MCT-09-1012.
Wu, W. I., Voegtli, W. C., Sturgis, H. L., Dizon, F. P., Vigers, G. P., & Brandhuber, B. J. (2010). Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS One, 5(9), e12913. https://doi.org/10.1371/journal.pone.0012913.
Zhang, X., Pickin, K. A., Bose, R., Jura, N., Cole, P. A., & Kuriyan, J. (2007). Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature, 450(7170), 741–744. https://doi.org/10.1038/nature05998.
Betzi, S., Alam, R., Martin, M., Lubbers, D. J., Han, H., Jakkaraj, S. R., Georg, G. I., & Schonbrunn, E. (2011). Discovery of a potential allosteric ligand binding site in CDK2. ACS Chemical Biology, 6(5), 492–501. https://doi.org/10.1021/cb100410m.
Kooistra, A. J., Kanev, G. K., van Linden, O. P. J., Leurs, R., de Esch, I. J. P., & de Graaf, C. (2016). KLIFS: A structural kinase-ligand interaction database. Nucleic Acids Research, 44(D1), D365–D371. https://doi.org/10.1093/nar/gkv1082.
van Linden, O. P., Kooistra, A. J., Leurs, R., de Esch, I. J., & de Graaf, C. (2014). KLIFS: A knowledge-based structural database to navigate kinase-ligand interaction space. Journal of Medicinal Chemistry, 57(2), 249–277. https://doi.org/10.1021/jm400378w.
Liao, J. J. (2007). Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. Journal of Medicinal Chemistry, 50(3), 409–424. https://doi.org/10.1021/jm0608107.
Zhao, Z., Liu, Q., Bliven, S., Xie, L., & Bourne, P. E. (2017). Determining cysteines available for covalent inhibition across the human kinome. Journal of Medicinal Chemistry, 60(7), 2879–2889. https://doi.org/10.1021/acs.jmedchem.6b01815.
Sordella, R., Bell, D. W., Haber, D. A., & Settleman, J. (2004). Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science, 305(5687), 1163–1167. https://doi.org/10.1126/science.1101637.
Gajiwala, K. S., Feng, J., Ferre, R., Ryan, K., Brodsky, O., Weinrich, S., Kath, J. C., & Stewart, A. (2013). Insights into the aberrant activity of mutant EGFR kinase domain and drug recognition. Structure, 21(2), 209–219. https://doi.org/10.1016/j.str.2012.11.014.
Chaikuad, A., Koch, P., Laufer, S. A., & Knapp, S. (2018). The cysteinome of protein kinases as a target in drug development. Angewandte Chemie (International Ed. in English), 57(16), 4372–4385. https://doi.org/10.1002/anie.201707875.
Zhao, Z., & Bourne, P. E. (2018). Progress with covalent small-molecule kinase inhibitors. Drug Discovery Today, 23(3), 727–735. https://doi.org/10.1016/j.drudis.2018.01.035.
Kim, S., Loo, A., Chopra, R., Caponigro, G., Huang, A., Vora, S., Parasuraman, S., Howard, S., Keen, N., Sellers, W., & Brain, C. (2014). Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6-Reactivating Rb in cancer. Molecular Cancer Therapeutics, 12(Suppl 11), PR02. https://doi.org/10.1158/1535-7163.targ-13-pr02.
Chen, P., Lee, N. V., Hu, W., Xu, M., Ferre, R. A., Lam, H., Bergqvist, S., Solowiej, J., Diehl, W., He, Y. A., Yu, X., Nagata, A., VanArsdale, T., & Murray, B. W. (2016). Spectrum and degree of CDK drug interactions predicts clinical performance. Molecular Cancer Therapeutics, 15(10), 2273–2281. https://doi.org/10.1158/1535-7163.mct-16-0300.
Dummer, R., Ascierto, P. A., Gogas, H. J., Arance, A., Mandala, M., Liszkay, G., Garbe, C., Schadendorf, D., Krajsova, I., Gutzmer, R., Chiarion-Sileni, V., Dutriaux, C., de Groot, J. W. B., Yamazaki, N., Loquai, C., Moutouh-de Parseval, L. A., Pickard, M. D., Sandor, V., Robert, C., & Flaherty, K. T. (2018). Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. The Lancet Oncology, 19(5), 603–615. https://doi.org/10.1016/s1470-2045(18)30142-6.
Corcoran, R. B., Andre, T., Atreya, C. E., Schellens, J. H. M., Yoshino, T., Bendell, J. C., Hollebecque, A., McRee, A. J., Siena, S., Middleton, G., Muro, K., Gordon, M. S., Tabernero, J., Yaeger, R., O’Dwyer, P. J., Humblet, Y., De Vos, F., Jung, A. S., Brase, J. C., Jaeger, S., Bettinger, S., Mookerjee, B., Rangwala, F., & Van Cutsem, E. (2018). Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discovery, 8(4), 428–443. https://doi.org/10.1158/2159-8290.CD-17-1226.
Maverakis, E., Cornelius, L., Bowen, G., Phan, T., Patel, F., Fitzmaurice, S., He, Y., Burrall, B., Duong, C., Kloxin, A., Sultani, H., Wilken, R., Martinez, S., & Patel, F. (2015). Metastatic melanoma—A review of current and future treatment options. Acta Dermato Venereologica, 95(5), 516–524. https://doi.org/10.2340/00015555-2035.
Bollag, G., Hirth, P., Tsai, J., Zhang, J., Ibrahim, P. N., Cho, H., Spevak, W., Zhang, C., Zhang, Y., Habets, G., Burton, E. A., Wong, B., Tsang, G., West, B. L., Powell, B., Shellooe, R., Marimuthu, A., Nguyen, H., Zhang, K. Y., Artis, D. R., Schlessinger, J., Su, F., Higgins, B., Iyer, R., D’Andrea, K., Koehler, A., Stumm, M., Lin, P. S., Lee, R. J., Grippo, J., Puzanov, I., Kim, K. B., Ribas, A., McArthur, G. A., Sosman, J. A., Chapman, P. B., Flaherty, K. T., Xu, X., Nathanson, K. L., & Nolop, K. (2010). Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature, 467(7315), 596–599. https://doi.org/10.1038/nature09454.
Tsai, J., Lee, J. T., Wang, W., Zhang, J., Cho, H., Mamo, S., Bremer, R., Gillette, S., Kong, J., Haass, N. K., Sproesser, K., Li, L., Smalley, K. S. M., Fong, D., Zhu, Y. L., Marimuthu, A., Nguyen, H., Lam, B., Liu, J., Cheung, I., Rice, J., Suzuki, Y., Luu, C., Settachatgul, C., Shellooe, R., Cantwell, J., Kim, S. H., Schlessinger, J., Zhang, K. Y. J., West, B. L., Powell, B., Habets, G., Zhang, C., Ibrahim, P. N., Hirth, P., Artis, D. R., Herlyn, M., & Bollag, G. (2008). Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proceedings of the National Academy of Sciences of the United States of America, 105(8), 3041–3046. https://doi.org/10.1073/pnas.0711741105.
Okamoto, K., Ikemori-Kawada, M., Jestel, A., von Konig, K., Funahashi, Y., Matsushima, T., Tsuruoka, A., Inoue, A., & Matsui, J. (2015). Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Medicinal Chemistry Letters, 6(1), 89–94. https://doi.org/10.1021/ml500394m.
Weisberg, E., Boulton, C., Kelly, L. M., Manley, P., Fabbro, D., Meyer, T., Gilliland, D. G., & Griffin, J. D. (2002). Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell, 1(5), 433–443. https://doi.org/10.1016/s1535-6108(02)00069-7.
Gotlib, J., Kluin-Nelemans, H. C., George, T. I., Akin, C., Sotlar, K., Hermine, O., Awan, F. T., Hexner, E., Mauro, M. J., Sternberg, D. W., Villeneuve, M., Huntsman Labed, A., Stanek, E. J., Hartmann, K., Horny, H. P., Valent, P., & Reiter, A. (2016). Efficacy and safety of midostaurin in advanced systemic mastocytosis. The New England Journal of Medicine, 374(26), 2530–2541. https://doi.org/10.1056/NEJMoa1513098.
Gibney, G. T., & Zager, J. S. (2013). Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies. Expert Opinion on Drug Metabolism and Toxicology, 9(7), 893–899. https://doi.org/10.1517/17425255.2013.794220.
Zhang, C., Spevak, W., Zhang, Y., Burton, E. A., Ma, Y., Habets, G., Zhang, J., Lin, J., Ewing, T., Matusow, B., Tsang, G., Marimuthu, A., Cho, H., Wu, G., Wang, W., Fong, D., Nguyen, H., Shi, S., Womack, P., Nespi, M., Shellooe, R., Carias, H., Powell, B., Light, E., Sanftner, L., Walters, J., Tsai, J., West, B. L., Visor, G., Rezaei, H., Lin, P. S., Nolop, K., Ibrahim, P. N., Hirth, P., & Bollag, G. (2015). RAF inhibitors that evade paradoxical MAPK pathway activation. Nature, 526(7574), 583–586. https://doi.org/10.1038/nature14982.
Zhao, Z., Xie, L., & Bourne, P. E. (2017). Insights into the binding mode of MEK type-III inhibitors. A step towards discovering and designing allosteric kinase inhibitors across the human kinome. PLoS One, 12(6), e0179936. https://doi.org/10.1371/journal.pone.0179936.
Long, G. V., Hauschild, A., Santinami, M., Atkinson, V., Mandalà, M., Chiarion-Sileni, V., Larkin, J., Nyakas, M., Dutriaux, C., Haydon, A., Robert, C., Mortier, L., Schachter, J., Schadendorf, D., Lesimple, T., Plummer, R., Ji, R., Zhang, P., Mookerjee, B., Legos, J., Kefford, R., Dummer, R., & Kirkwood, J. M. (2017). Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. New England Journal of Medicine, 377(19), 1813–1823. https://doi.org/10.1056/NEJMoa1708539.
Ayeni, D., Politi, K., & Goldberg, S. B. (2015). Emerging agents and new mutations in EGFR-mutant lung cancer. Clinical Cancer Research, 21(17), 3818–3820. https://doi.org/10.1158/1078-0432.CCR-15-1211.
Patel, H., Pawara, R., Ansari, A., & Surana, S. (2017). Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance. European Journal of Medicinal Chemistry, 142, 32–47. https://doi.org/10.1016/j.ejmech.2017.05.027.
Jia, Y., Yun, C. H., Park, E., Ercan, D., Manuia, M., Juarez, J., Xu, C., Rhee, K., Chen, T., Zhang, H., Palakurthi, S., Jang, J., Lelais, G., DiDonato, M., Bursulaya, B., Michellys, P. Y., Epple, R., Marsilje, T. H., McNeill, M., Lu, W., Harris, J., Bender, S., Wong, K. K., Janne, P. A., & Eck, M. J. (2016). Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature, 534(7605), 129–132. https://doi.org/10.1038/nature17960.
Zhao, Z., Wu, H., Wang, L., Liu, Y., Knapp, S., Liu, Q., & Gray, N. S. (2014). Exploration of type II binding mode: A privileged approach for kinase inhibitor focused drug discovery? ACS Chemical Biology, 9(6), 1230–1241. https://doi.org/10.1021/cb500129t.
Juchum, M., Gunther, M., & Laufer, S. A. (2015). Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resistance Updates, 20, 12–28. https://doi.org/10.1016/j.drup.2015.05.002.
Bean, J., Brennan, C., Shih, J. Y., Riely, G., Viale, A., Wang, L., Chitale, D., Motoi, N., Szoke, J., Broderick, S., Balak, M., Chang, W. C., Yu, C. J., Gazdar, A., Pass, H., Rusch, V., Gerald, W., Huang, S. F., Yang, P. C., Miller, V., Ladanyi, M., Yang, C. H., & Pao, W. (2007). MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences of the United States of America, 104(52), 20932–20937. https://doi.org/10.1073/pnas.0710370104.
Wu, Y. L., Zhang, L., Kim, D. W., Liu, X., Lee, D. H., Yang, J. C., Ahn, M. J., Vansteenkiste, J. F., Su, W. C., Felip, E., Chia, V., Glaser, S., Pultar, P., Zhao, S., Peng, B., Akimov, M., & Tan, D. S. W. (2018). Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of endothelial growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. Journal of Clinical Oncology, 36(31), 3101–3109. https://doi.org/10.1200/JCO.2018.77.7326.
Acknowledgments
Thanks to Peng Wu for useful insights and corrections when reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhao, Z., Bourne, P.E. (2020). Overview of Current Type I/II Kinase Inhibitors. In: Shapiro, P. (eds) Next Generation Kinase Inhibitors. Springer, Cham. https://doi.org/10.1007/978-3-030-48283-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-48283-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48282-4
Online ISBN: 978-3-030-48283-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)