Abstract
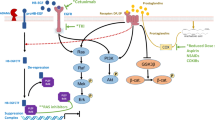
The importance of prostaglandin E2 in cancer progression is well established, but research on its role in cancer has so far mostly been focused on epithelial cancer in adults while the knowledge about the contribution of prostaglandin E2 to childhood malignancies is limited. Neuroblastoma, an extracranial solid tumor of the sympathetic nervous system, mainly affects young children. Patients with tumors classified as high-risk have poor survival despite receiving intensive treatment, illustrating a need for new treatments complimenting existing ones. The basis of neuroblastoma treatment e.g. chemotherapy and radiation therapy, target the proliferating genetically unstable tumor cells leading to treatment resistance and relapses. The tumor microenvironment is an avenue, still to a great extent, unexplored and lacking effective targeted therapies. Cancer-associated fibroblasts is the main source of prostaglandin E2 in neuroblastoma contributing to angiogenesis, immunosuppression and tumor growth. Prostaglandin E2 is formed from its precursor arachidonic acid in a two-step enzymatic reaction. Arachidonic acid is first converted by cyclooxygenases into prostaglandin H2 and then further converted by microsomal prostaglandin E synthase-1 into prostaglandin E2. We believe targeting of microsomal prostaglandin E synthase-1 in cancer-associated fibroblasts will be an effective future therapeutic strategy in fighting neuroblastoma.
Per Kogner and Per-Johan Jakobsson contributed equally to this work.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Cohen EG, Almahmeed T, Du BH et al (2003) Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma. Clin Cancer Res 9(9):3425–3430
Larsson K, Kock A, Idborg H et al (2015) COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc Natl Acad Sci U S A 112(26):8070–8075. https://doi.org/10.1073/pnas.1424355112
van Rees BP, Sivula A, Thorén S et al (2003) Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer 107(4):551–556. https://doi.org/10.1002/ijc.11422
Yoshimatsu K, Golijanin D, Paty PB et al (2001) Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res 7(12):3971–3976
Yoshimatsu K, Altorki NK, Golijanin D et al (2001) Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res 7(9):2669–2674
Pai R, Soreghan BA, Szabo IL et al (2002) Prostaglandin E-2 directly promotes human colon cancer growth by triggering activation of ERK2, c-fos gene and cell proliferation. Gastroenterology 122(4):A240–A240
Sheng H, Shao J, Washington MK, DuBois RN (2001) Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem 276(21):18075–18081. https://doi.org/10.1074/jbc.M009689200
Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN (1998) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58(2):362–366
Pai R, Szabo IL, Soreghan BA et al (2001) PGE(2) stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun 286(5):923–928. https://doi.org/10.1006/bbrc.2001.5494
Buchanan FG, Wang D, Bargiacchi F, DuBois RN (2003) Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem 278(37):35451–35457. https://doi.org/10.1074/jbc.M302474200
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188(1):21–28. https://doi.org/10.4049/jimmunol.1101029
Obermajer N, Wong JL, Edwards RP et al (2012) PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Investig 41(6–7):635–657. https://doi.org/10.3109/08820139.2012.695417
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216. https://doi.org/10.1038/nrc1014
Johnsen JI, Kogner P, Albihn A, Henriksson MA (2009) Embryonal neural tumours and cell death. Apoptosis 14(4):424–438. https://doi.org/10.1007/s10495-009-0325-y
Cohn SL, Pearson AD, London WB et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol 27(2):289–297. https://doi.org/10.1200/JCO.2008.16.6785
Carén H, Kryh H, Nethander M et al (2010) High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc Natl Acad Sci U S A 107(9):4323–4328. https://doi.org/10.1073/pnas.0910684107
Ladenstein R, Potschger U, Pearson ADJ et al (2017) Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 18(4):500–514. https://doi.org/10.1016/S1470-2045(17)30070-0
Moreno L, Vaidya SJ, Pinkerton CR et al (2013) Long-term follow-up of children with high-risk neuroblastoma: the ENSG5 trial experience. Pediatr Blood Cancer 60(7):1135–1140. https://doi.org/10.1002/pbc.24452
Friedman R (2016) Drug resistance in cancer: molecular evolution and compensatory proliferation. Oncotarget 7(11):11746–11755. https://doi.org/10.18632/oncotarget.7459
Kurtova AV, Xiao J, Mo Q et al (2015) Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517(7533):209–213. https://doi.org/10.1038/nature14034
Borriello L, Seeger RC, Asgharzadeh S, DeClerck YA (2015) More than the genes, the tumor microenvironment in neuroblastoma. Cancer Lett 380(1):304–314. https://doi.org/10.1016/j.canlet.2015.11.017
Matthay KK, Maris JM, Schleiermacher G et al (2016) Neuroblastoma. Nat Rev Dis Primers 2:Article:16078. https://doi.org/10.1038/nrdp.2016.78
Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B (1999) Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A 96(13):7220–7225
Subbaramaiah K, Yoshimatsu K, Scherl E et al (2004) Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1. J Biol Chem 279(13):12647–12658. https://doi.org/10.1074/jbc.M312972200
Uracz W, Uracz D, Olszanecki R, Gryglewski RJ (2002) Interleukin 1beta induces functional prostaglandin E synthase in cultured human umbilical vein endothelial cells. J Physiol Pharmacol 53(4 Pt 1):643–654
Xiao L, Ornatowska M, Zhao G et al (2012) Lipopolysaccharide-induced expression of microsomal prostaglandin E synthase-1 mediates late-phase PGE2 production in bone marrow derived macrophages. PLoS One 7(11):e50244. https://doi.org/10.1371/journal.pone.0050244
Donnini S, Finetti F, Terzuoli E et al (2012) EGFR signaling upregulates expression of microsomal prostaglandin E synthase-1 in cancer cells leading to enhanced tumorigenicity. Oncogene 31(29):3457–3466. https://doi.org/10.1038/onc.2011.503
Xue X, Shah YM (2013) Hypoxia-inducible factor-2alpha is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis 34(1):163–169. https://doi.org/10.1093/carcin/bgs313
Murakami M, Nakashima K, Kamei D et al (2003) Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem 278(39):37937–37947. https://doi.org/10.1074/jbc.M305108200
Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I (2000) Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem 275(42):32775–32782. https://doi.org/10.1074/jbc.M003504200
Tanikawa N, Ohmiya Y, Ohkubo H et al (2002) Identification and characterization of a novel type of membrane-associated prostaglandin E synthase. Biochem Biophys Res Commun 291(4):884–889. https://doi.org/10.1006/bbrc.2002.6531
Jania LA, Chandrasekharan S, Backlund MG et al (2009) Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E-2 biosynthesis. Prostaglandins Other Lipid Mediat 88(3–4):73–81. https://doi.org/10.1016/j.prostaglandins.2008.10.003
Hara S (2017) Prostaglandin terminal synthases as novel therapeutic targets. Proc Jpn Acad Ser B Phys Biol Sci 93(9):703–723. https://doi.org/10.2183/pjab.93.044
Cano LQ, Lavery DN, Sin S et al (2015) The co-chaperone p23 promotes prostate cancer motility and metastasis. Mol Oncol 9(1):295–308. https://doi.org/10.1016/j.molonc.2014.08.014
Simpson NE, Gertz J, Imberg K, Myers RM, Garabedian MJ (2012) Research resource: enhanced genome-wide occupancy of estrogen receptor alpha by the cochaperone p23 in breast cancer cells. Mol Endocrinol 26(1):194–202. https://doi.org/10.1210/me.2011-1068
Yu R, Xiao L, Zhao GQ, Christman JW, van Breemen RB (2011) Competitive enzymatic interactions determine the relative amounts of prostaglandins E-2 and D-2. J Pharmacol Exp Ther 339(2):716–725. https://doi.org/10.1124/jpet.111.185405
Sugimoto Y, Narumiya S (2007) Prostaglandin E receptors. J Biol Chem 282(16):11613–11617. https://doi.org/10.1074/jbc.R600038200
Nakanishi M, Rosenberg DW (2013) Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 35(2):123–137. https://doi.org/10.1007/s00281-012-0342-8
Harris SG, Padilla J, Koumas L, Ray D, Phipps RP (2002) Prostaglandins as modulators of immunity. Trends Immunol 23(3):144–150. https://doi.org/10.1016/S1471-4906(01)02154-8
Snijdewint FGM, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML (1993) Prostaglandin-E(2) differentially modulates cytokine secretion profiles of human T-helper lymphocytes. J Immunol 150(12):5321–5329
Wang D, Dubois RN (2010) Eicosanoids and cancer. Nat Rev Cancer 10(3):181–193. https://doi.org/10.1038/nrc2809
Obermajer N, Kalinski P (2012) Key role of the positive feedback between PGE(2) and COX2 in the biology of myeloid-derived suppressor cells. Oncoimmunology 1(5):762–764. https://doi.org/10.4161/onci.19681
Baratelli F, Lin Y, Zhu L et al (2005) Prostaglandin E-2 induces FOXP3 gene expression and T regulatory cell function in human CD4(+) T cells. J Immunol 175(3):1483–1490. https://doi.org/10.4049/jimmunol.175.3.1483
Miao J, Lu X, Hu YF et al (2017) Prostaglandin E-2 and PD-1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget 8(52):89802–89810. https://doi.org/10.18632/oncotarget.21155
Goto T, Herberman RB, Maluish A, Strong DM (1983) Cyclic-Amp as a mediator of prostaglandin-E-induced suppression of human natural-killer cell-activity. J Immunol 130(3):1350–1355
Kalinski P, Hilkens CMU, Snijders A, Snijdewint FGM, Kapsenberg ML (1997) IL-12-deficient dendritic cells, generated in the presence of prostaglandin E-2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 159(1):28–35
Liu LX, Ge DX, Ma L et al (2012) Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol 7(7):1091–1100. https://doi.org/10.1097/JTO.0b013e3182542752
Wan SS, Zhao ED, Kryczek I et al (2014) Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147(6):1393–1404. https://doi.org/10.1053/j.gastro.2014.08.039
Öhlund D, Elyada E, Tuveson D (2014) Fibroblast heterogeneity in the cancer wound. J Exp Med 211(8):1503–1523. https://doi.org/10.1084/jem.20140692
Guo XY, Oshima H, Kitmura T, Taketo MM, Oshima M (2008) Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem 283(28):19864–19871. https://doi.org/10.1074/jbc.M800798200
Wang XY, Klein RD (2007) Prostaglandin E-2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog 46(11):912–923. https://doi.org/10.1002/mc.20320
Carlson LM, Rasmuson A, Idborg H et al (2013) Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis 34(5):1081–1088. https://doi.org/10.1093/carcin/bgt009
Asgharzadeh S, Salo JA, Ji L et al (2012) Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 30(28):3525–3532. https://doi.org/10.1200/JCO.2011.40.9169
Hashimoto O, Yoshida M, Koma Y et al (2016) Collaboration of cancer-associated fibroblasts and tumour-associated macrophages for neuroblastoma development. J Pathol 240(2):211–223. https://doi.org/10.1002/path.4769
Borriello L, Nakata R, Sheard MA et al (2017) Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Cancer Res 77(18):5142–5157. https://doi.org/10.1158/0008-5472.CAN-16-2586
Pietras K, Östman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331. https://doi.org/10.1016/j.yexcr.2010.02.045
Zeine R, Salwen HR, Peddinti R et al (2009) Presence of cancer-associated fibroblasts inversely correlates with Schwannian stroma in neuroblastoma tumors. Mod Pathol 22(7):950–958. https://doi.org/10.1038/modpathol.2009.52
Kock A, Larsson K, Bergqvist F et al (2018) Inhibition of microsomal prostaglandin E synthase-1 in cancer-associated fibroblasts suppresses neuroblastoma tumor growth. EBioMedicine 32:84–92. https://doi.org/10.1016/j.ebiom.2018.05.008
Olajide OA, Velagapudi R, Okorji UP, Sarker SD, Fiebich BL (2014) Picralima nitida seeds suppress PGE(2) production by interfering with multiple signalling pathways in IL-1 beta-stimulated SK-N-SH neuronal cells. J Ethnopharmacol 152(2):377–383. https://doi.org/10.1016/j.jep.2014.01.027
Wendeburg L, de Oliveira ACP, Bhatia HS, Candelario-Jalil E, Fiebich BL (2009) Resveratrol inhibits prostaglandin formation in IL-1 beta-stimulated SK-N-SH neuronal cells. J Neuroinflammation 6:Artn 26. https://doi.org/10.1186/1742-2094-6-26
Rasmuson A, Kock A, Fuskevåg OM et al (2012) Autocrine prostaglandin E2 signaling promotes tumor cell survival and proliferation in childhood neuroblastoma. PLoS One 7(1):e29331. https://doi.org/10.1371/journal.pone.0029331
Johnsen JI, Lindskog M, Ponthan F et al (2004) Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res 64(20):7210–7215. https://doi.org/10.1158/0008-5472.CAN-04-1795
Tsutsumimoto T, Williams P, Yoneda T (2014) The SK-N-AS human neuroblastoma cell line develops osteolytic bone metastases with increased angiogenesis and COX-2 expression. J Bone Oncol 3(3–4):67–76. https://doi.org/10.1016/j.jbo.2014.10.002
Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM (1997) Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J 16(11):2985–2995. https://doi.org/10.1093/emboj/16.11.2985
Allen CP, Tinganelli W, Sharma N et al. (2015) DNA damage response proteins and oxygen modulate prostaglandin E-2 growth factor release in response to low and high LET ionizing radiation. Front Oncol 5:UNSP 260. https://doi.org/10.3389/fonc.2015.00260
Huang Q, Li F, Liu XJ et al (2011) Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17(7):860–U231. https://doi.org/10.1038/nm.2385
Trifan OC, Durham WF, Salazar VS et al (2002) Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res 62(20):5778–5784
Greenhough A, Smartt HJM, Moore AE et al (2009) The COX-2/PGE(2) pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30(3):377–386. https://doi.org/10.1093/carcin/bgp014
Hangai S, Ao T, Kimura Y et al (2016) PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc Natl Acad Sci U S A 113(14):3844–3849. https://doi.org/10.1073/pnas.1602023113
Liu QL, Yuan WQ, Tong DL et al (2016) Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget 7(19):28235–28246. https://doi.org/10.18632/oncotarget.8595
Pang LY, Hurst EA, Argyle DJ (2016) Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int 2016:1–11. Artn 2048731. https://doi.org/10.1155/2016/2048731
Ponthan F, Wickström M, Gleissman H et al (2007) Celecoxib prevents neuroblastoma tumor development and potentiates the effect of chemotherapeutic drugs in vitro and in vivo. Clin Cancer Res 13(3):1036–1044. https://doi.org/10.1158/1078-0432.CCR-06-1908
Kaneko M, Kaneko S, Suzuki K (2009) Prolonged low-dose administration of the cyclooxygenase-2 inhibitor celecoxib enhances the antitumor activity of irinotecan against neuroblastoma xenografts. Cancer Sci 100(11):2193–2201. https://doi.org/10.1111/j.1349-7006.2009.01280.x
Davis TW, O’Neal JM, Pagel MD et al (2004) Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2-derived prostaglandin E-2, a survival factor for tumor and associated vasculature. Cancer Res 64(1):279–285. https://doi.org/10.1158/0008-5472.Can-03-1168
Hennequart M, Pilotte L, Cane S et al (2017) Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res 5(8):695–709. https://doi.org/10.1158/2326-6066.CIR-16-0400
Hou W, Sampath P, Rojas JJ, Thorne SH (2016) Oncolytic virus-mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell 30(1):108–119. https://doi.org/10.1016/j.ccell.2016.05.012
Zelenay S, van der Veen AG, Bottcher JP et al (2015) Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162(6):1257–1270. https://doi.org/10.1016/j.cell.2015.08.015
Casteels-Van Daele M, Van Geet C, Wouters C, Eggermont E (2000) Reye syndrome revisited: a descriptive term covering a group of heterogeneous disorders. Eur J Pediatr 159(9):641–648
Pugliese A, Beltramo T, Torre D (2008) Reye’s and Reye’s-like syndromes. Cell Biochem Funct 26(7):741–746. https://doi.org/10.1002/cbf.1465
Franco VI, Lipshultz SE (2015) Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young 25:107–116. https://doi.org/10.1017/S1047951115000906
Pawelzik SC, Uda NR, Spahiu L et al (2010) Identification of key residues determining species differences in inhibitor binding of microsomal prostaglandin E synthase-1. J Biol Chem 285(38):29254–29261. https://doi.org/10.1074/jbc.M110.114454
Leclerc P, Idborg H, Spahiu L et al (2013) Characterization of a human and murine mPGES-1 inhibitor and comparison to mPGES-1 genetic deletion in mouse models of inflammation. Prostaglandins Other Lipid Mediat 107:26–34. https://doi.org/10.1016/j.prostaglandins.2013.09.001
Ozen G, Gomez I, Daci A et al (2017) Inhibition of microsomal PGE synthase-1 reduces human vascular tone by increasing PGI2: a safer alternative to COX-2 inhibition. Br J Pharmacol 174:4087–4098. https://doi.org/10.1111/bph.13939
Koeberle A, Werz O (2015) Perspective of microsomal prostaglandin E-2 synthase-1 as drug target in inflammation-related disorders. Biochem Pharmacol 98(1):1–15. https://doi.org/10.1016/j.bcp.2015.06.022
Psarra A, Nikolaou A, Kokotou MG, Limnios D, Kokotos G (2017) Microsomal prostaglandin E-2 synthase-1 inhibitors: a patent review. Expert Opin Ther Pat 27(9):1047–1059. https://doi.org/10.1080/13543776.2017.1344218
Bergqvist F, Ossipova E, Idborg H et al (2019) Inhibition of mPGES-1 or COX-2 results in different proteomic and lipidomic profiles in A549 lung cancer cells. Front Pharmacol. https://doi.org/10.3389/fphar.2019.00636
Acknowledgements
This work was supported by grants from the Swedish Childhood Cancer Foundation, the Cancer Society in Stockholm, the Swedish Cancer Society, the Swedish Foundation for Strategic Research (www.nnbcr.se), Märta and Gunnar V Philipson Foundation and Karolinska Institutet Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Larsson, K., Kock, A., Kogner, P., Jakobsson, PJ. (2019). Targeting the COX/mPGES-1/PGE2 Pathway in Neuroblastoma. In: Honn, K., Zeldin, D. (eds) The Role of Bioactive Lipids in Cancer, Inflammation and Related Diseases. Advances in Experimental Medicine and Biology, vol 1161. Springer, Cham. https://doi.org/10.1007/978-3-030-21735-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-21735-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21636-8
Online ISBN: 978-3-030-21735-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)