Abstract
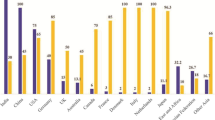
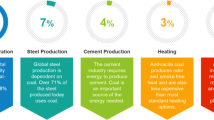
Coal-fueled global electricity production is estimated to be 44% by 2030 producing 750 million tons of fly ash, a waste product in combustion, and disposed of in huge amounts on landfills. The improper disposal of such anthropogenic waste-coal fly ash has resulted in environmental issues and needs the sustainable waste management. There are various components of fly ash, cenosphere being one of the most researched ones as to develop recycling methods for fly ash cenospheres. The smart properties of the cenosphere such as lightweight, high thermal resistance, chemical inertness, high compressive strength, less water absorption capacity, etc., make fly ash cenosphere ideal for many industrial and target-specific applications. Cenosphere has found major applications in the construction industry, as fillers to various polymeric composites, shielding to electromagnetic radiation, microwave absorption, oil well drilling material, heat, fire, and sound insulation. The present review focuses on the global generation of coal fly ash, formation, separation of fly ash cenosphere, and summarizing the various modification techniques to develop the functionalized properties on fly ash cenosphere. It also covers the basic concepts related to fly ash cenosphere and its mode of utilization in high-end applications for energy and environmental issues.
Similar content being viewed by others
References
Chavez-Valdez A, Arizmendi-Morquecho A, Vargas G, Almanza JM, Alvarez-Quintana J (2011) Ultra-low thermal conductivity thermal barrier coatings from recycled fly-ash cenospheres. Acta Mater 59(6):2556–2562. https://doi.org/10.1016/j.actamat.2011.01.011
Yao ZT et al (2015) A comprehensive review on the applications of coal fly ash. Earth Sci Rev 141:105–121. https://doi.org/10.1016/j.earscirev.2014.11.016
Wang S (2008) Application of solid ash based catalysts in heterogeneous catalysis. Environ Sci Technol 42(19):7055–7063
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36(3):327–363. https://doi.org/10.1016/j.pecs.2009.11.003
Blissett RS, Rowson NA (2012) Review article: a review of the multi-component utilisation of coal fly ash. Fuel 97:1–23. https://doi.org/10.1016/j.fuel.2012.03.024
Cho H, Oh D, Kim K (2005) A study on removal characteristics of heavy metals from aqueous solution by fly ash. J Hazard Mater 127:187–195. https://doi.org/10.1016/j.jhazmat.2005.07.019
Nyale SM, Eze CP, Akinyeye RO, Gitari WM, Akinyemi SA, Fatoba OO, Petrik LF (2014) The leaching behaviour and geochemical fractionation of trace elements in hydraulically disposed weathered. J Environ Sci Health Part A Toxic Hazard Subst Environ 49:233–242. https://doi.org/10.1080/10934529.2013.838929
Bhangare RC, Tiwari M, Ajmal PY, Sahu SK, Pandit GG (2014) Distribution of natural radioactivity in coal and combustion residues of thermal power plants. J Radioanal Nucl Chem 300:17–22. https://doi.org/10.1007/s10967-014-2942-3
Fomenko EV, Anshits NN, Solovyov LA, Mikhaylova OA, Anshits AG (2013) Composition and morphology of fly ash cenospheres produced from the combustion of Kuznetsk coal. Energy Fuel 27:5440
Vassilev SV, Vassileva CG (1996) Mineralogy of combustion wastes from coal-fired power stations. Fuel Process Technol 47(96):261–280
Goodarzi F (2006) Characteristics and composition of fly ash from Canadian coal-fired power plants. Fuel 85:1418–1427. https://doi.org/10.1016/j.fuel.2005.11.022
Sokol EV, Maksimova NV, Volkova NI (2000) Hollow silicate microspheres from fly ashes of the Chelyabinsk brown coals ž South Urals, Russia. Fuel Process Technol 67:35
Li Y, Wu H (2012) Ash cenosphere from solid fuels combustion. Part 1: an investigation into its formation mechanism using pyrite as a model fuel. Energy Fuel 26:130–137
Ranjbar N, Kuenzel C (2017) Cenospheres: a review. Fuel 207:1–12. https://doi.org/10.1016/j.fuel.2017.06.059
Anshits NN, Mikhailova OA, Salanov AN, Anshits AG (2010) Chemical composition and structure of the shell of fly ash non-perforated cenospheres produced from the combustion of the Kuznetsk coal (Russia). Fuel 89(8):1849–1862. https://doi.org/10.1016/j.fuel.2010.03.049
Kolay PK, Bhusal S (2014) Recovery of hollow spherical particles with two different densities from coal fly ash and their characterization. Fuel 117:118–124. https://doi.org/10.1016/j.fuel.2013.09.014
Self A (1995) Particle size-density relation and cenosphere content of coal fly ash. Fuel 74(4):522–529
Hirajima T, Petrus HTBM, Oosako Y, Nonaka M, Sasaki K, Ando T (2010) Recovery of cenospheres from coal fly ash using a dry separation process: separation estimation and potential application. Int J Miner Process 95(1–4):18–24. https://doi.org/10.1016/j.minpro.2010.03.004
Shapiro M, Galperin V (2005) Air classification of solid particles: a review. Chem Eng Process Process Intensif 44:279–285. https://doi.org/10.1016/j.cep.2004.02.022
Vassilev SV, Menendez R, Diaz-somoano M, Martinez-tarazona MR (2004) Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 83:585–603. https://doi.org/10.1016/j.fuel.2003.10.003
Fomenko EV et al (2015) Characterization of fly ash cenospheres produced from the combustion of Ekibastuz coal. Energy Fuel. https://doi.org/10.1021/acs.energyfuels.5b01022
Pikulin IV, Potemkin GA, Redyushev SA (2008) Formation processes and main properties of hollow aluminosilicate microspheres in fly ash from thermal power stations. Solid Fuel Chem 42(2):107–119. https://doi.org/10.3103/S0361521908020110
Fomenko EV et al (2012) Influence of the composition and structure of the glass crystalline shell of cenospheres. Glas Phys Chem 38(2):218–227. https://doi.org/10.1134/S1087659612020046
Anshits NN, Vereshchagina TA, Bayukov OA, Salanov AN, Anshits AG (2005) The nature of nanoparticles of crystalline phases in cenospheres and morphology of their shells. Glas Phys Chem 31(3):306–315. Proceedings of the Topical Meeting of the European Ceramic Society “Nanoparticles, Nanostructures, and Nanocomposites”
Ngu L, Wu H, Zhang D (2007) Characterization of ash cenospheres in fly ash from Australian. Energy Fuel 31(9):3437–3445
Chalivendra VB, Shukla A, Parameswaran V (2003) Processing and mechanical characterization of lightweight polyurethane composites. J Mater Sci 38:1631–1643
Tiwari M, Shukla SP, Mohan D, Bhargava DS, Kisku GC (2015) Modified cenospheres as an adsorbent for the removal of disperse dyes. Adv Environ Chem 2015:1
Shukla S, Seal S, Akesson J, Oder R, Carter R, Rahman Z (2001) Study of mechanism of electroless copper coating of fly-ash cenosphere particles. Appl Surf Sci 181:35–50
Wang W, Zhai J, Li Q (2015) Synthesis of buoyant metal-coated fly ash cenosphere and its excellent catalytic performance in dye degradation. J Colloid Interface Sci 444:10–16. https://doi.org/10.1016/j.jcis.2014.12.059
Yu X, Shen Z (2009) The electromagnetic shielding of Ni films deposited on cenosphere particles by magnetron sputtering method. J Magn Magn Mater 321(18):2890–2895. https://doi.org/10.1016/j.jmmm.2009.04.040
Shukla S, Seal S, Rahaman Z, Scammon K (2002) Electroless copper coating of cenospheres using silver nitrate activator. Mater Lett 57(1):151–156. https://doi.org/10.1016/S0167-577X(02)00722-X
Aixiang Z, Weihao X, Jian X (2005) Electroless Ni-Co-P coating of cenospheres using [Ag(NH3) 2]+ activator. Mater Lett 59(4):524–528. https://doi.org/10.1016/j.matlet.2004.10.041
Aixiang Z, Weihao X, Jian X (2005) Electroless Ni-P coating of cenospheres using silver nitrate activator. Surf Coat Technol 197(2–3):142–147. https://doi.org/10.1016/j.surfcoat.2005.01.009
Pang J et al (2012) Preparation and characterization of electroless Ni-Fe-P alloy films on fly ash cenospheres. Powder Technol 226:246–252. https://doi.org/10.1016/j.powtec.2012.04.055
Zeng A, Hu K, Li L (2012) Electroless Ni-P coating of cenospheres using copper sulfate activator. Adv Mater Res 463–464:375–379. https://doi.org/10.4028/www.scientific.net/AMR.463-464.375
Cao XG, Ren H, Zhang HY (2015) Preparation and microwave shielding property of silver-coated carbonyl iron powder. J Alloys Compd 631:133–137. https://doi.org/10.1016/j.jallcom.2015.01.103
Shishkin A, Hussainova I, Kozlov V, Lisnanskis M, Leroy P, Lehmhus D (2018) Metal-coated cenospheres obtained via magnetron sputter coating: a new precursor for syntactic foams. J Miner Met Mater Soc. https://doi.org/10.1007/s11837-018-2886-0
Shishkin A, Drozdova M, Kozlov V, Hussainova I, Lehmhus D (2017) Vibration-assisted sputter coating of cenospheres: a new approach for realizing Cu-based metal matrix syntactic foams. Metals (Basel) 7(1). https://doi.org/10.3390/met7010016
Meng X, Li D, Shen X, Liu W (2010) Preparation and magnetic properties of nano-Ni coated cenosphere composites. Appl Surf Sci 256:3753–3756. https://doi.org/10.1016/j.apsusc.2010.01.019
Meng X, Shen X (2012) Preparation of FeCo-, FeNi- and NiCo-alloy coated cenosphere composites by heterogeneous precipitation. Particuology 10(3):334–338. https://doi.org/10.1016/j.partic.2011.02.012
Li D, Zhou J, Shen X, Liu W (2010) Fabrication of magnetic nanosized γ-FeNi-coated ceramic core–shell microspheres by heterogeneous precipitation and thermal reduction. Particuology 8(3):257–261. https://doi.org/10.1016/j.partic.2009.05.008
Surolia PK, Tayade RJ, Jasra RV (2010) TiO2-coated cenospheres as catalysts for photocatalytic degradation of methylene blue, p-nitroaniline, n-decane, and n-tridecane under solar irradiation. Ind Eng Chem Res 49(19):8908–8919. https://doi.org/10.1021/ie100388m
Lu Z et al (2013) Performance of a novel TiO2 photocatalyst based on the magnetic floating fly-ash cenospheres for the purpose of treating waste by waste. Chem Eng J 225:34–42. https://doi.org/10.1016/j.cej.2013.03.077
Huo P et al (2010) H2O2 modified surface of TiO2/fly-ash cenospheres and enhanced photocatalytic activity on methylene blue. Desalination 263(1–3):258–263. https://doi.org/10.1016/j.desal.2010.06.067
Huo P, Yan Y, Li S, Li H, Huang W (2009) Preparation and characterization of Cobalt Sulfophthalocyanine/TiO2/fly-ash cenospheres photocatalyst and study on degradation activity under visible light. Appl Surf Sci 255(15):6914–6917. https://doi.org/10.1016/j.apsusc.2009.03.014
Mathapati M, Doddamani M, Ramesh MR (2017) High-temperature erosive behavior of plasma sprayed Cr3C2-NiCr/cenosphere coating. J Mater Eng Perform. https://doi.org/10.1007/s11665-018-3226-9
Wadatkar SS, Shende DZ, Wasewar KL (2020) Synthesis of copper-coated ceramic core–shell cenosphere in fluidized bed reactor using H2/N2 gas for thermal reduction. Mater Today Proc 29:850–856. https://doi.org/10.1016/j.matpr.2020.05.022
Jha N, Badkul A, Mondal DP, Das S, Singh M (2011) Sliding wear behavior of aluminum syntactic foam: a comparison with Al–10 wt % SiC composites. Tribiology Int 44(3):220–231. https://doi.org/10.1016/j.triboint.2010.10.004
Rohatgi PK, Gupta N, Schultz BF, Luong DD (2011) The synthesis, compressive properties, and applications of metal matrix syntactic foams. JOM 63:36
Fu W, Liu S, Fan W, Yang H, Pang X (2007) Hollow glass microspheres coated with CoFe2O4 and its microwave absorption property. J Magn Magn Mater 316:54–58. https://doi.org/10.1016/j.jmmm.2007.03.201
Xu X et al (2011) Adsorption of fluoride from aqueous solution on magnesia-loaded fly ash cenospheres. Desalination 272(1–3):233–239. https://doi.org/10.1016/j.desal.2011.01.028
Xu Z, Yu XZ, Shen ZG (2013) Coating nano-nickel film on cenospheres by magnetic sputtering method. Adv Mater Res 750–752:2044–2047. https://doi.org/10.4028/www.scientific.net/AMR.750-752.2044
Kim S, Choe W, Choi J, Jeong J (2019) Preparation and characterization of silver-coated magnetic microspheres prepared by a modified electroless plating process. Powder Technol 342:301–307. https://doi.org/10.1016/j.powtec.2018.09.094
Song J et al (2017) Photocatalytic enhancement of floating photocatalyst: layer-by-layer hybrid carbonized chitosan and Fe-N- codoped TiO2 on fly ash cenospheres. Appl Surf Sci 391:236–250. https://doi.org/10.1016/j.apsusc.2016.04.021
Mushtaq F, Zahid M, Bhatti IA, Nasir S, Hussain T (2019) Possible applications of coal fly ash in wastewater treatment. J Environ Manag 240:27–46. https://doi.org/10.1016/j.jenvman.2019.03.054
Makode V, Shende DZ, Wasewar KL, Wanjari SP (2016) Process development for coating of metal and metal oxides on hollow ceramic microspheres. Intellectual Property Rights, Indian Patents, 201621034195
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Shende, D.Z., Wasewar, K.L., Wadatkar, S.S. (2021). Target-Specific Applications of Fly Ash Cenosphere as Smart Material. In: Kharissova, O.V., Martínez, L.M.T., Kharisov, B.I. (eds) Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications. Springer, Cham. https://doi.org/10.1007/978-3-030-11155-7_207-1
Download citation
DOI: https://doi.org/10.1007/978-3-030-11155-7_207-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11155-7
Online ISBN: 978-3-030-11155-7
eBook Packages: Springer Reference Chemistry and Mat. ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics