Abstract
The giant sable antelope Hippotragus niger variani is the most widely recognised representative of Angolan biodiversity, owing to its endemic status, rarity and physical attributes. One of the last large mammals to be described in Africa, it is confined to the upper Cuanza basin, in central Angola. Studies on the biology of giant sable were mostly conducted in the 1970s, but ongoing efforts using modern tools such as DNA analyses, GPS tracking, camera trapping and satellite imagery are improving our knowledge. Past explanations for the extent of the isolation and relationships with other sable populations have been controversial. Molecular studies have only recently made significant contributions to interpret the evolutionary history of giant sable. Although much pursued by hunters during the first half of the twentieth century, the conservation needs of giant sable were recognised early on, with the proclamation of two protected areas and the setting in place of strict regulations. Park management and efficient protection was enforced in the 1960s, but these protected areas were abandoned soon after the country’s independence, leading to population crashes and interspecific hybridization, which left the subspecies on the verge of extinction. The giant sable is currently the main focus of a conservation programme supervised by the Angolan Government that is successfully promoting its recovery.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Cangandala
- Conservation
- Cuanza
- Evolutionary history
- Extinction
- Hybridisation
- Luando
- Population collapse
- Trap cameras
Introduction and Background
Having captured the imagination of naturalists and the general public for over one hundred years, the giant sable antelope Hippotragus niger variani is the undisputed icon of Angola’s natural heritage (Fig. 17.1). Its cultural significance extends from local totemic status among resident communities (where it is known as ‘kolo’ or ‘sumbakaloko’) to global recognition as an antelope symbol and flagship for conservation. Soon after its discovery, the giant sable was elevated to a high pedestal among the hunting community as one of the most sought after trophy prizes, and fuelled the lust of big game hunters from all over the world. In Angola, the giant sable was the first animal to receive full legal protection and was soon embraced as an icon during colonial rule. Since Angola’s independence in 1975, its status has been reinforced. The importance of the current unanimous recognition of the giant sable as a national symbol should not be underestimated, constituting a key factor uniting Angola’s people regardless of their different ethnic groups, religious beliefs or political ideologies, and thus contributing to social cohesion and national pride.
Observations on the biology of giant sable were reported soon after its scientific description by Thomas (1916), resulting from the many expeditions undertaken to collect material for museums in Europe and the United States of America. Of particular importance were very detailed morphological observations, with taxonomic implications and accurate ecological descriptions, made by Gilbert Blaine (1922). In addition, behavioural observations were made by trophy hunters (e.g. Statham 1922; Gray 1930, 1933; Powell-Cotton 1932; Curtis 1933), while concerns expressed by the subspecies’ discoverer himself led to timely conservation interventions (Varian 1953). For a long time these reports would remain as the most reliable sources of knowledge on the taxonomy and biology of giant sable, although several other publications addressed its conservation status (Harper 1945; Heim 1954; Newton da Silva 1958). In the late 1950s and during the following decade, the first systematic efforts focusing on giant sable addressed its biology and related conservation issues, and were undertaken by Portuguese researchers working for the Centro de Zoologia da Junta de Investigação do Ultramar or Instituto de Investigação Científica de Angola (e.g. Frade 1958, 1967; Frade and Sieiro 1960; Sieiro 1962; Crawford-Cabral 1965, 1966, 1969, 1970). Later, Huntley (1972) made important contributions on conservation planning for giant sable while adding ecological insights. Published in 1972, the book A Palanca Real (Silva 1972) provided a comprehensive and appealing photographic compilation, including a few ecological and behavioural observations. The researcher who became inextricably linked with the giant sable, the famous biologist Richard Estes, spent one full year studying the giant sable in Luando Nature Strict Reserve between 1969 and 1970. Estes is still the most relevant contributor to the current knowledge on the biology of the species (e.g. Estes and Estes 1970, 1972, 1974). Another invaluable source is the book A Certain Curve of Horn (Walker 2004), in which the author gives a detailed and vivid account of the various explorations, studies and conservation initiatives around the giant sable, a history spanning over 100 years. In recent years, molecular studies have addressed giant sable looking into phylogenetic relationships, genetic diversity or hybridisation (e.g. Pitra et al. 2006; Jansen van Vuuren et al. 2010; Vaz Pinto et al. 2015, 2016). Currently, comprehensive ongoing research is being developed on the giant sable and addressing a wide range of topics, including evolutionary history, biology and conservation (Vaz Pinto 2018).
Scientific Discovery
The giant sable was not discovered and described until the twentieth century, but two intriguing and obscure previous records are worth mentioning. The oldest proven giant sable material is a single horn, named ‘The Florence Horn’ after the museum in Italy where it was deposited in 1873 (Walker 2004; Vaz Pinto 2018). Nothing is known about the provenance details of the Florence Horn. Although recognised early on as extraordinary, and suspected later to have been obtained from the Angolan relic population (Thomas 1916; Walker 2004), only recently has a molecular study provided convincing evidence of it being a giant sable horn (Vaz Pinto 2018). The Florence Horn may, however, have been preceded by a skull collected by the famous Austrian botanist Friedrich Welwitsch in Angola between 1853 and 1861, and classified by Bocage as Hippotragus niger (Bocage 1878, 1890; Thomas 1916). As with so many other priceless biological material from Angola, this specimen was tragically lost in the fire that destroyed the Museu Bocage in 1978, and it was never possible to attribute it to subspecies, even though the reported length of the horns suggest that it was a giant sable. The specimen was stated as having been collected by Welwitsch inland from Moçâmedes (Bocage 1878, 1890; Thomas 1916; Hill and Carter 1941). The exact collecting locality remains in doubt, but it is known that Welwitsch collected in the Malanje region (Crawford-Cabral and Mesquitela 1989), which could have given him access to giant sable.
The discovery and scientific description of this taxon had to wait another half-century, and followed the efforts of the chief engineer overseeing the construction of the Benguela Railway, the British citizen Frank Varian (Varian 1953; Walker 2004). The first mention of a sable with extraordinary horns from the Cuanza district was made by Varian in 1909 and simply based on one photograph and witness accounts, but it was met with disbelief in Europe (Varian 1953; Walker 2004). The first material was secured in 1911, but it was only in 1916 that skulls and skins were shipped by Varian to the British Museum and led to the formal description of Hippotragus niger variani (Thomas 1916), thus honouring the discoverer.
Description
The giant sable is a large, compact and muscular antelope carrying massive scimitar-shaped horns. The original description was based on a giant sable skull with horns that measured 57 inches in length along the curvature and 11 inches in circumference at the base (Thomas 1916). Horn length is a distinctive trademark for giant sable as they are usually above 50 and often above 60 inches, while bulls from all other sable antelope populations only very rarely surpass 50 inches (Halse 1998; Vaz Pinto 2018).
The size of horns alone made the type specimen stand out, but equally striking was the darker face, in which unlike other sable, the ante-orbital white spots are not connected by a white streak to the sides of the muzzle (Thomas 1916). These features proved consistent as more specimens were analysed, but other peculiarities were revealed to be distinctive, as described in detail by Gilbert Blaine (1922). Very clear differences have been found in the skulls, both structurally and from measurements, and these helped to sustain a claim for elevating giant sable to specific status (Blaine 1922). Giant sable have longer and narrower forefaces and less prominent forehead compared to typical sables, and relatively small ears in adult males, while the neck in mature bulls is short, massive, oval in section and wedge shaped (Blaine 1922). Other published measurements on both skulls (Groves and Grubb 2011) and teeth (Klein 1974) have relied on very small sample sizes, and therefore added little to Blaine’s observations.
The combination of skull and body build, crowned by the massive arched horns, must have greatly contributed to the ‘giant’ epithet attributed to this taxon, but as no specimen was ever weighted and few were measured (Blaine 1922; Harper 1945; Estes 1982, 2013), doubts remain regarding the size of giant sable in comparison with other sable antelope populations. Giant sable, particularly bulls, have often been considered as much larger in size and weight than other sable (Blaine 1922; Statham 1922; Harper 1945), but this view was not shared by Estes (1982, 2013). Nevertheless, recent observations made on immobilised animals suggest that mature bulls may be at least heavier than other sable subspecies (Vaz Pinto Unpublished Data).
Sexual dimorphism is pronounced in sable, but appears to be more extreme in giant sable (Fig. 17.2), possibly as a by-product of their sedentary nature and the mesic environmental in which they occur (Estes 2013; Vaz Pinto 2018). The distinct characteristics observed in males and female giant sable is obvious in body size, horn length, and pelage colouration. Various physical and colouration features were described in great detail by Blaine (1922). In short, mature giant sable bulls are glossy black with white bellies, white facial markings, reddish hocks and yellowish back to the ears (Blaine 1922; Frade and Sieiro 1960; Estes 2013). In females, the black colour of bulls is replaced by brown tones which have been described as differently as bright golden chestnut (Blaine 1922), light to dark brown-chocolate (Silva 1972) or deep chestnut to blackish brown (Groves and Grubb 2011). Female colouration has been used to assist differentiation among sable subspecies (Groves and Grubb 2011), but pelage colouration of giant sable females is variable irrespective of age (Vaz Pinto Unpublished Data).
Taxonomy
While examining the Angolan material, Oldfield Thomas, the mammal curator of the British Museum, was so impressed by some peculiarities and obvious differences when comparing Varian’s material with ordinary sable that he considered the possibility of awarding specific status to the giant sable (Thomas 1916; Blaine 1922; Walker 2004). Subsequently, and having assembled additional material, Gilbert Blaine (1922) found it justifiable to recognise giant sable as a full species on its own Hippotragus variani, a view that was followed by some authors (e.g. Harper 1945). Nevertheless, Blaine’s claim eventually fell into disuse and the giant sable became widely accepted as one of four to five subspecies of sable antelope (e.g. Hill and Carter 1941; Ansell 1972; Groves and Grubb 2011; Estes 2013).
With the advent of molecular tools, the taxonomic placement of giant sable could be revisited. The first molecular studies to include giant sable samples were based on small mitochondrial fragments and reported paraphyly for giant sable with respect to sable antelope across the southern parts of their range. These findings cast doubt on the recognition of H. n. variani as a valid subspecies (Mathee and Robinson 1999; Pitra et al. 2006). The results, in combination with the fact that sable collected in western Zambia often present similar facial markings, were further interpreted as blurring the differences between giant and western Zambian sable antelope (Wessels 2007). However, clear genetic differences exist between these western Zambian sable and giant sable, confirming that the giant sable represents a distinct mitochondrial evolutionary lineage (Jansen van Vuuren et al. 2010).
Much progress has recently been achieved allowing the clarification of the taxonomic status of giant sable, following more ambitious molecular efforts that included the use of mitogenomics and nuclear markers (Vaz Pinto 2018). The full sequencing of mitochondria on a large dataset pooled from across the full range of sable distribution, concluded that giant sable constitutes a reciprocally monophyletic group, one of six geographically discrete sable clusters (Vaz Pinto 2018). Confirming previous suggestions (Pitra et al. 2006; Jansen van Vuuren et al. 2010), the giant sable maternal lineage proved to be more closely related to some west Tanzanian lineages, than to those found elsewhere in southern Africa (Vaz Pinto 2018). A population approach resorting to 57 autosomal microsatellites applied to an even more comprehensive dataset showed giant sable as being the most divergent of five clearly identified and geographically coherent populations (Fig. 17.3, Vaz Pinto 2018). The molecular results combining mitogenomics and nuclear markers provide compelling evidence to support the uniqueness of giant sable, and are therefore concordant with its unequivocal recognition as a separate endemic taxon (Vaz Pinto 2018).
Sable Antelope populations (and sub-species) as determined through nuclear DNA analysis (Vaz Pinto 2018). Eastern Sable Hippotragus niger roosevelti; Tanzanian Sable (possibly H. n. subsp. nov); Zambian Sable H. n. kirki; Angolan Sable H. n. variani; Southern Sable H. n. niger
Evolutionary History
The origin of giant sable has remained intriguing and subject to different interpretations, and only recently by resorting to modern molecular tools, a more robustly sustained and coherent picture is emerging. Attempting to explain the rarity of giant sable and its oddly restricted distribution, Huntley (1972) argued it not to be a recent artefact driven by anthropogenic causes, but rather a result of very specific habitat requirements that forced this population to a long-standing confinement in central Angola. Other authors have suggested alternative views such as the possibility of H. n. kirkii from western Zambian having had at some point a continuous distribution into the Angolan plateau before receding and a subpopulation becoming isolated in the Cuanza Basin (Crawford-Cabral and Veríssimo 2005; Wessels 2007).
The first molecular studies to include giant sable, based on small mitochondrial fragments and limited sampling (Mathee and Robinson 1999; Pitra et al. 2006) found H. n. variani samples to cluster within other sables, and results suggested recent connectivity with other southern African populations. The suggestion that giant sable may share a closer ancestry with some (but not all of) west Tanzanian lineages rather than with the geographically closer west Zambian populations (Pitra et al. 2006; Jansen van Vuuren et al. 2010) was difficult to interpret and explain. It was speculated that central Angola and southern Tanzania could have been colonised from a common source population (Pitra et al. 2006). Furthermore, a tentative date for this split was set at approximately 200,000 years ago (Pitra et al. 2006). In an effort to disentangle this phylogenetic riddle, Groves and Grubb (2011) suggested that H. n. variani could have migrated from Angola to southern Tanzania and hybridised with H. n. roosevelti, but this scenario is difficult to justify and, if anything, would complicate things even further.
A more recent molecular study based on mitogenomics revealed the phylogeographic patterns that may have shaped the evolutionary history of sable antelope, influenced by a complex interplay between Pleistocene climatic oscillations and geomorphological features (Vaz Pinto 2018). The study also provided credible time estimations for time splitting events. It was hypothesised that during the dry climatic period of the Elster Glaciation, corresponding to the Marine Isotopic Stage (MIS) 6 and estimated at approximately between 130,000 and 186,000 years ago, an ancestral sable lineage evolved in Central Congo and separated from two other lineages in eastern and southern Africa (Vaz Pinto 2018). When the climate became warmer and receding savannas were replaced by forests, at around 120,000 years ago, the Central Congo lineage would have split into an Angolan lineage and another that ended up in the rift region (Vaz Pinto 2018). Subsequently, the Angolan lineage may have since evolved confined to the Cuanza river basin and adapted to particular habitat conditions (Vaz Pinto 2018). The population approach using autosomal nuclear markers confirmed the uniqueness of the giant sable population, with low levels of gene flow for a long time, and as a result being genetically very distinctive (Vaz Pinto 2018). In addition it was possible to genetically differentiate the populations from Luando and Cangandala, even though the genetic signal may have been enhanced by an extreme recent bottleneck affecting the latter (Vaz Pinto 2018). It seems possible that the giant sable evolved in isolation in central Angola since the beginning of the late Pleistocene until the present day, and exposed to relatively rare male-mediated gene flow events with neighbouring sable populations (Vaz Pinto 2018).
Habitat and Ecology
As with other sable races, the giant sable is a specialist of miombo, a type of woodland and mesic savanna that occurs on poor dystrophic soils dominated by trees from the genus Brachystegia, Julbernardia and Isoberlinia (Estes 2013, Fig. 17.4). Giant sable are ecotone species, showing a preferential use of the edge between woodland and grassland (Estes and Estes 1974; Estes 2013). A peculiar feature in the gently undulating giant sable region are vast termite-infested open savannas covered by fire-prone geoxyle vegetation, and known by the local name of anharas (Barbosa 1970; Estes and Estes 1974; Zigelski et al. 2019). A mosaic of woodland and anharas seems to constitute the prime habitat for giant sable herds both in Luando Strict Nature Reserve (LSNR) and Cangandala National Park (CNP) (Estes and Estes 1974). It has also been suggested that the presence of these anharas, characterised by unique vegetation types and influenced by poor soils and unique local climatic conditions, may explain the current pattern of giant sable distribution (Vaz Pinto 2018). Giant sable are also water-dependant, and the availability of water, in streams or water holes, during the dry season, is a key component determining habitat value and can become a limiting factor affecting their distribution patterns (Estes and Estes 1974).
Giant sable are herbivores and mostly selective grazers, with preference for perennial grasses such as Brachiaria, Digitaria, Panicum or Setaria spp., and typically biting off only the tender outer portion of the plants (Estes and Estes 1974). Although predominantly grazers, they also browse frequently, in particular the locally abundant shrub species Diplorhynchus condylocarpon which seems to be favoured by giant sable all year round (Estes and Estes 1974; Vaz Pinto Unpublished Data), to the point of being referred by locals as ‘palanca bush’ (Statham 1922). Also browsed often are the tree Julbernardia paniculata, and the dwarf shrubs Mucana stans, Cryptosepalum maraviense and Dolichus sp (Statham 1922; Crawford-Cabral 1970; Silva 1972; Estes and Estes 1974; Vaz Pinto 2018). In addition it has been found that giant sable resort often to geophagy, eating soil excavated on some ancient termite mounds, a habit likely evolved as a consequence of very low nutrient soils (Estes and Estes 1974; Baptista et al. 2013).
In common with most other social antelopes, giant sable are gregarious and are structured according to three social classes: the breeding or nursery herds, bachelor groups, and territorial bulls (Estes and Estes 1974; Estes 2013). The matriarchal herd, composed of breeding cows, calves and young, constitute the main unit (Estes and Estes 1974; Estes 2013). Numbers and composition of giant sable breeding herds change seasonally and sometimes even daily, and different average figures have been obtained by various authors, usually ranging from eight to 24 animals (Blaine 1922; Crawford-Cabral 1966, 1970; Estes and Estes 1974; Vaz Pinto Unpublished Data). The nursery herds are sedentary and tend to perpetuate their home ranges across generations (Estes and Estes 1974; Estes 2013). Young males will be tolerated within the herd until around 3 years of age, before dispersing where after they either initiate solitary life or temporarily join other males to form bachelor groups (Estes and Estes 1974). Around their sixth year of age, bulls become territorial, and will demarcate their own territory by scrapping, defecating and bush-thrashing with their horns (Estes and Estes 1974). Dominant bulls typically display aggressive behaviour towards intruders, exerting domination by physical intimidation and chasing, and only exceptionally will the confrontation lead to a serious fight (Estes and Estes 1974).
Giant sable are seasonal breeders. The rutting coincides with miombo springtime and has been stated to start around late August (Estes and Estes 1974), although recent observations suggest the mating season to peak in September and October (Vaz Pinto 2018). The gestation likely follows what has been found for other sable populations, estimated at 8.5–9 months (Wilson and Hirst 1977). The calving season for giant sable matches the onset of the dry season, peaking during a 2-month period between May and July (Estes and Estes 1972), but off-season calving is not unusual (Estes and Estes 1974; Vaz Pinto Unpublished Data). As the calving season approaches, the breeding herds tend to break and the most heavily pregnant cows become isolated (Estes and Estes 1974). Giant sable are ‘hiders’, meaning that females will calve alone and hide their calves, attending them at irregular intervals for several days or weeks, before re-joining the herd with their offspring, and forming crèches with other calves of similar age (Estes and Estes 1974; Estes 2013).
Giant sable herds have been found to make use of home ranges of varying size, with one of two giant sable herds monitored for 1 year in Luando Strict Nature Reserve staying within 12 km2 while the other moved a distance of 15 km to use different areas in the dry and rainy seasons, raising the size of the annual home range of one of those groups to 40 km2 (Pedrosa 1971; Estes and Estes 1974). The same authors found home range size to be affected by food availability and seasonality (Estes and Estes 1974). Herds will tend to break up after the onset of rains, and move from the anharas into the woodlands (Estes and Estes 1974). During the wettest periods, sables will avoid waterlogged areas such as floodplains, and spend most of the time on high ground within the woodland (Crawford-Cabral 1970; Estes and Estes 1974). Nevertheless, recent data obtained with GPS transmitters, have found relatively little seasonal variation, and yet a huge contrast in home range size, ranging from 14 to 110 km2 measured by Minimum Convex Polygon (MCP) (Vaz Pinto 2018). The daily movements of herds is reported to be modest, typically varying from one to two km (Estes and Estes 1974), and this finding is consistent with GPS data (Vaz Pinto Unpublished Data). In general, herd movement patterns can be summarised as concentrations in open areas during the dry season, followed by group partition as the rain starts and confinement of smaller stable groups in wooded parts of their range, and then increased movements towards the end of the rains and further group fragmentation prior to the calving season (Estes and Estes 1974). Different herds will not overlap in home ranges and are frequently separated by several km of seemingly suitable habitat (Estes and Estes 1974). Sable bulls have been reported to hold relatively small territories separated from neighbours by 1 to 3 km apart, while spending most of their time within 3–4 km2, and are able to expand their area to at least 10–12 km2 when accompanying breeding herds (Estes and Estes 1974). However, preliminary data obtained from GPS monitoring of several giant sable bulls over a period of 5 years suggest a very different spatial use, as bulls tend to move a lot more than previously thought, and with overlapping territories ranging above 200 km2 measured as MCP’s (Vaz Pinto 2018).
Historical Distribution and Abundance
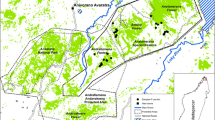
Soon after the description of H. n. variani, it was assumed that they only occurred within the Cuanza watershed, and especially confined to the lowlands between the upper Cuanza and its tributary the Luando (Blaine 1922; Statham 1922; Hill and Carter 1941; Varian 1953). This region, also known as the ‘land between two rivers’ (Walker 2004), is a narrow strip of land stretching across 200 km oriented NW – SE, and spanning up to 60 km at its widest. Most of the hunters and naturalists exploring the region in search of giant sable entered the sable lands from the Benguela Railroad, heading northwards and thus focusing on the southern region within the species' distribution (Walker 2004). Nevertheless, one of the earliest giant sable explorations, by Col. Statham, entered from the north, and traversed across the land between the two rivers on foot, and was able to confirm the presence of giant sable up to the confluence of the Luando and Cuanza rivers (Statham 1922). Effectively limited by the two massive rivers to the north, to the south giant sable seemed to be contained by three features: swamps, an inland escarpment, and a desolate territory to the east crossed by the railway and known as the ‘Hungry Country’ (Varian 1953; Walker 2004). Within the ‘land between two rivers’, sable appear to prefer the Luando and Lingoio sub-basins while avoiding the remaining Cuanza drainage (Blaine 1922; Statham 1922; Vaz Pinto Unpublished Data). The land between two rivers was set aside as a conservation area in 1938, first as the Giant Sable Reserve, and since 1955 as Luando Strict Nature Reserve (Huntley 1971), and covering approximately 828,000 hectares.
The possible existence of a giant sable population north of the Luando River, near the village of Cangandala, was first suggested by Statham (1922). This was based on trophies acquired by Statham from a Portuguese settler. However, Statham seems to have been deliberately misled by local tribal chiefs who denied that giant sable occurred elsewhere than to the south of the Luando River (Statham 1922; Walker 2004). More than 30 years would pass before the existence of a second, albeit much smaller, sable population north of the Luando was eventually confirmed near Cangandala between the Cuque and Cuije rivers (Frade 1958), and a mere 50 km south of Malanje town. As result of this find, Cangandala Strict Nature Reserve was proclaimed in 1963, and elevated to a National Park status in 1970. The occurrence of giant sable outside the boundaries of Luando Reserve and Cangandala National Park was often claimed but never proven conclusively. Witness accounts mentioned sable to be present between the Cuanza River and its western tributary the Cutato (Blaine 1922), but by the 1970’s their persistence in the region was at best doubtful (Estes and Estes 1974; Huntley Unpublished Data). Other unconfirmed records were reported from the ‘Hungry Country’ and from the areas that lie in between Cangandala National Park and Luando Strict Nature Reserve (Estes and Estes 1974; Crawford-Cabral and Veríssimo 2005; Huntley Unpublished Data). Two of the most geographically extreme giant sable records, falling outside the Cuanza basin, were from Quibala in Cuanza-Sul, and from Baixa de Cassanje in Lundas, but only males were reported (Estes and Estes 1974; Huntley Unpublished Data). A third case was a trophy obtained near Lupire in Cuando Cubango in 1966 and described by the collector as a very old lonely bull in poor condition (Francisco Sousa Machado pers. comm.). The trophy would have become the world record if accepted as a typical sable (Halse 1998; Wessels 2007), but a recent molecular study has established it as giant sable (Vaz Pinto 2018). Other reports of giant sables from as far from Luando as Katanga in the Democratic Republic of Congo or western Zambia (Schouteden 1947; Wessels 2007) are likely erroneous and lack molecular support (Ansell 1972; Jansen van Vuuren et al. 2010; Vaz Pinto 2018).
As bulls may disperse far and unpredictably, it is fair to conclude that the historical distribution of giant sable has remained well contained within the Cuanza Basin, and likely centred in Luando Reserve (Estes and Estes 1974), with relatively small population pockets reaching Cangandala National Park and surrounding areas.
The first estimations of giant sable populations were based on limited knowledge of the species distribution and lacked reliable quantifiable data, suggesting the total numbers to reach a few hundred individuals. Based on guestimates provided by Portuguese naturalists, numbers were set at less than 750–800 (Harper 1945) or at around 700 (Heim 1954). By partially surveying approximately one third of Luando Reserve, 159 giant sable were counted (Frade and Sieiro 1960; Newton da Silva 1970), leading Fernando Frade to suggest that the total number could be even less than 500 (Frade 1958, 1967). As conservation measures increased and biologists renewed their interest in the giant sable, subsequent efforts raised the estimations to range between 1500 and 2500 animals (Crawford-Cabral 1970; Huntley 1972, 1973). Nevertheless, and as pointed out by Richard Estes, these estimations should be taken with care as they were not based on actual counts (Estes and Estes 1972, 1974). In the early 1970s a few aerial counts were attempted by plane and helicopter, but the results did not contribute much for determining the population size (Estes and Estes 1970, 1974, Pedrosa 1971). With the bulk of giant sable present in Luando Reserve, the population in Cangandala NP was estimated to range between 100 and 150 animals (Crawford-Cabral 1970; Huntley 1973; João Serôdio Unpublished Data). The existing estimations suggest therefore an average giant sable density of 0.0025 sable/ha.
Collapse, Rediscovery, Hybridisation
The civil war that ravaged Angola following the country’s independence in 1975 had a dramatic impact on the populations of giant sable. As result of the war, management was abandoned in protected areas and infrastructure destroyed, while accounts reported on the widespread killing of giant sable (Walker 2004). A brief visit made in 1982 by Richard Estes, then chairman of the IUCN Antelope Specialist Group, was able to confirm various herds of giant sable that were still being monitored by a park warden in Cangandala (Estes 1982). But by then the Luando Reserve had been taken over by UNITA (União Nacional para a Independência Total de Angola) military and could not be surveyed by Estes, and the reports were worrisome (Walker 2004). Soon after Estes’ visit the warden was forced to flee Cangandala as UNITA took control of the park and in Luando the rangers that stayed behind were killed. When a short peace allowed a national biodiversity assessment to be carried out in 1992, the situation was stated as mostly unknown in spite of recent sightings (Huntley and Matos 1992), and as the armed conflict resumed and grew in intensity many questioned if the survival of giant sable was even a realistic possibility (Walker 2004). Efforts to find the species were put on hold, and could only be implemented when peace and security were restored in the bush (Walker 2004).
In November 2001, a few months before the end of the war, the Kissama Foundation, a local biodiversity Non-Governmental Organisation, made a flight over Cangandala National Park on a large military helicopter without results (Walker 2004). A subsequent and more ambitious effort was carried out in August 2002, soon after the war ended, by the Kissama Foundation and again with strong support from the Angolan military. On this occasion, Cangandala was subject to a one-day survey by a large party on foot, and a couple of flights on a military helicopter were done over Luando Reserve (Walker 2004). No animals were seen from the air, but brief observations were reported in Cangandala of what could have been giant sable, although they could not be substantiated (Walker 2004). Ground surveys were then implemented in Cangandala by the Centro de Estudos e Investigação Científica (CEIC) of the Catholic University of Angola starting in 2003, and in the following year surveys extended into Luando Reserve where an aerial survey was also performed. No giant sable were seen, but Hippotragus dung was collected in both protected areas. In 2004, a monitoring programme with trap cameras was also initiated. Finally, in early 2005, it was possible to prove the survival of giant sable when DNA extracted from dung samples revealed that the mitochondria was typical of H. n. variani, and trap camera photographs showed sable herds in Cangandala (Pitra et al. 2006).
Although giant sable had proved resilient enough to persist in Cangandala National Park, the situation proved to be much worse than anticipated. As the trap camera records increased and additional ground observations were made in Cangandala, an alarming scenario emerged: only one herd of giant sable was present, no sable bulls could be found, a roan bull was seen escorting the sable herd, and based on morphological features alone, calves and young animals appeared to be hybrids (Vaz Pinto 2007). The possibility of interspecific hybridisation was subsequently confirmed with modern molecular tools (Vaz Pinto et al. 2016). The occurrence and extent of this phenomenon was unexpected, and allowed for a quite unprecedented study among mammals, as it was possible to document in detail the underlying mechanisms that led to such an extreme outcome (Vaz Pinto et al. 2016). By 2009, the last sable herd in Cangandala included nine old pure females and nine hybrids (Vaz Pinto et al. 2016). In spite of being naturally sympatric and with deep independent evolutionary histories, roan and sable were not only able to hybridise but also produced at least two confirmed second generation hybrids (Vaz Pinto et al. 2016).
In Luando, a combination of ground surveys, trap camera monitoring and four aerial surveys with helicopter between 2009 and 2016, suggested that as result of the war, giant sable had been extirpated from approximately 75% of their historical range, but a few herds did manage to survive in the remaining area, and may have totalled around 100 animals at the end of the war in 2002 (Vaz Pinto 2018).
The extreme population crashes that affected the giant sable populations, were further unravelled by an extensive genetic study using mitogenomes and nuclear DNA, applied on a very extensive dataset that included dozens of modern samples and pre-war material obtained from natural history collections in museums around the world (Vaz Pinto 2018). The population collapse in Cangandala, caused a severe loss of heterozygosity that ranks among the lowest ever recorded in mammals (Vaz Pinto 2018). In Luando, the heterozygosity was only moderately reduced as result of the bottleneck, but the loss of mitochondrial diversity was extreme, when 11 haplotypes were found in samples dating from the early twentieth century and a single haplotype became fixed in the extant population (Vaz Pinto 2018). Such a pattern is consistent with a scenario in which at least two subpopulations coexisted in Luando with gene flow maintained by male dispersal, before the more ancient nucleus that used to function as repository of maternal diversity became extinct during the war (Vaz Pinto 2018).
Conservation
Being an endemic population, restricted to the north-central Angolan plateau, and extremely rare, the giant sable has always been a species of conservation concern, firstly listed in the 1933 London Convention for the Preservation of Fauna and Flora, as a Class A species worthy of absolute formal protection (Walker 2004). It also featured as Endangered in the IUCN Red List of Threatened Species since its creation in 1964, with the status revised to Critically Endangered in 1996 and maintained since then (IUCN 2017). The giant sable is also included in Appendix I of the Convention on International Trade of Endangered Species (CITES) since its creation in 1975.
The earliest known giant sable conservation policies, in the form of regional hunting bans, were adopted by the District Governor of Moxico in 1913 just prior to the formal species description, and by the high commissioner for the colony of Angola, General Norton de Matos in 1922 (Varian 1953; Walker 2004). On both occasions, these bans intended to counteract excessive hunting of giant sable, and must be credited to the vision and perseverance of Frank Varian, without whom the species may not have lasted long (Walker 2004). This however, did not prevent many trophy hunters from travelling to Angola to shoot giant sable in the 1920s and 1930s (Walker 2004). Following the listing as a Class A protected species by the London Convention of 1933, giant sable hunting and the possession of trophies was prohibited in 1934 (Heim 1954).
Nevertheless, these regulations were poorly enforced, and many specimens were still being collected by foreign hunters, Portuguese traders and local communities (Harper 1945). In 1938 the ‘Land between Two Rivers’ was demarcated as a hunting reserve, and named Reserva da Caça do Luando, thus laying the ground for the implementation of future integrated conservation policies. Recommendations were made by the International Union for the Conservation of Nature and Natural Resources (IUCN) for the creation of a National Park (Heim 1954), and in response the colonial government upgraded the category of Luando from a hunting reserve to a strict nature reserve in 1955 (Huntley 1971). In the same year, a new law regulating hunting and nature conservation in Angola underlined the giant sable as priority species worthy of full protection, and regulations published in 1957 criminalised the killing of giant sable, setting the fine at 100,000 escudos (Frade and Sieiro 1960), which would be equivalent to more than US $10,000 at current value. Although giant sable conservation finally started to be taken seriously and hunting had been almost terminated, one last trophy hunting expedition was authorised in 1961 following negotiations at the highest level (Agundis 1965; Vaz Pinto 2018). Conservation measures for the giant sable were discussed in the late 1950s (Newton da Silva 1958; Frade 1958; Frade and Sieiro 1960), while for the first time the need to protect the small pocket population found near the town of Cangandala was addressed (Frade 1958). The recognition of this second population would lead to the creation of Cangandala Strict Nature Reserve in 1963, and then elevated to a National park in 1970 (Huntley 1971).
By the early 1970s the giant sable was relatively well protected and benefited from conservation management practices in both Cangandala and Luando (Pedrosa 1971; Huntley 1973; Estes and Estes 1974). This was achieved in spite of relatively moderate budgets and small management teams, comprising two senior rangers and seven assistant-rangers in Luando, and one senior ranger with four assistant rangers in Cangandala (Huntley 1971). As the direct harvest of giant sable was much reduced and virtually eliminated with increased enforcement of protection and management, other threats gained in perceived relevance, particularly habitat deterioration due to slash and burn agriculture (Crawford-Cabral 1970; Huntley 1972; Estes and Estes 1974). Luando Reserve had a human resident population estimated at 18,000 in 1971 in addition to 800 people living inside Cangandala National Park, mostly resorting to tree-cutting to plant manioc, and thus negatively affecting the natural miombo woodland (Huntley 1971; Pedrosa 1971; Estes and Estes 1974). The possible relocation of human populations, conversion of agricultural practices, and even translocation of giant sable to safe havens was then suggested (Huntley 1972, 1973; Estes and Estes 1974). Nevertheless, such conservation concerns would soon become irrelevant.
During the Angolan civil war the situation deteriorated rapidly, and if conservation measures were still in place in Cangandala until 1982 (Estes 1982), soon after this all management and protection ceased across the giant sable areas. Conservation initiatives could only be reinstated in recent years, following the arrival of peace and the location of the last surviving population pockets (Vaz Pinto 2018). In the absence of formal management in Luando and Cangandala, the Giant Sable Conservation Project was launched in 2003 by the Catholic University of Angola, and since 2010 has been led by the Kissama Foundation. The Giant Sable Project has been assisting the Angolan Government in the implementation of conservation practices and management in both protected areas. Members of resident communities were trained and appointed as conservation agents, and some have already been transferred to the park management as government rangers. In Cangandala National Park, the Giant Sable Project has rehabilitated park infrastructure, deployed equipment and built fences (Vaz Pinto 2018). Extraordinary conservation measures were adopted in 2009 to tackle the hybridisation crisis in Cangandala National Park, leading to translocations, sterilisation of hybrids and the constitution of a breeding nucleus (Vaz Pinto et al. 2016).
The most critical issue that has affected the giant sable in recent years, causing the populations to crash and risking compromising their recovery, is widespread uncontrolled poaching driven by the bushmeat trade (Vaz Pinto 2018). Although giant sables do not appear to be specifically targeted by poachers, they are still shot at, but the most pervasive and negative impact is the large scale use of snares and foot traps that are having a huge toll, affecting mostly young females and immature sables (Vaz Pinto 2018).
The Giant Sable Project has installed a network of trap cameras covering a good part of the reserves, which has allowed regular monitoring and the identification of individuals, and was instrumental in detecting and documenting the hybridisation phenomenon in Cangandala (Vaz Pinto et al. 2016; Vaz Pinto 2018). Between 2009 and 2016, a total of 74 giant sable and nine hybrids were darted and marked, 65 of them released with tracking collars, which included 32 GPS collars, allowing much increased surveillance power and the gathering of knowledge on the biology of the species (Vaz Pinto 2018).
The Angolan Government has been boosting law enforcement measures, and in 2016 the fine for killing one giant sable was increased to a value equivalent to roughly US$100,000, although no one has been prosecuted for such an offense in recent decades. Currently, the conservation of giant sable is understandably focused on anti-poaching, relying on an increase of surveillance, close monitoring of animals and strengthening park management. By 2018, the giant sable populations had recovered to around 70 animals in Cangandala National Park and an estimated 150 in Luando.
The Way Forward
The unquestionable importance of securing the future of a critically endangered taxon, which also happens to be a flagship species and a national icon, must frame current and future activities. In addition to the obvious need to implement more effective law enforcement measures and enhance park management through infrastructure rehabilitation and staff recruitment and training, some specific issues deserving consideration include erecting new fences and making relocations to recover parts of the historical giant sable distribution.
As tools ultimately benefitting giant sable conservation and management, several lines of research can be developed, either as completely new approaches, or by building on previous work. A study of the food preferences of giant sable is a crucial topic which can now be addressed in detail with modern molecular tools, complemented with remote tracking of movements. Studies on the use of other local resources may also prove critical, such as water and natural salt licks. A better understanding of factors that affect the breeding success and calf mortality, is also needed. An epidemiological study is required, including a study on parasites potentially affecting the giant sable, and a monitoring programme should be implemented and extended to other species and domestic animals in the region. The impact of frequent dry-season fires, and how they reflect on vegetation and on the movements of giant sable should be assessed, and the use of fire as a management tool should be explored. The fact that many giant sable have been collared with GPS satellite transmitters and more will probably be in the future, opens up unique opportunities to develop research on the spatial use of herds and bulls, address territoriality, use of local resources, breeding and response to extrinsic factors. Existing molecular tools should continue to be employed to address individual identification, parameters of genetic diversity, with clear application on the management of existing populations. Comparisons with other subspecies and historical giant sable material, and development of more advance molecular tools such as with genomics, will also greatly improve our knowledge, and may assist future breeding efforts, and help preserve some critical and unique genetic features of this magnificent antelope.
References
Agundis TM (1965) El Llamado de la Montaña, Viajes de Cacería, Angola-Tanzania-Alaska. Rustica Editorial, Mexico
Ansell WFH (1972) Part 15: order Artiodactyla. In: Meester JAJ, Setzer HW (eds) The mammals of Africa: an identification manual. Smithsonian Institution Press, Washington, DC, pp 1–93
Baptista SL, Pinto PV, Freitas MDC et al (2013) Geophagy by African ungulates: the case of the critically endangered giant sable antelope of Angola (Hippotragus niger variani). Afr J Ecol 51(1):139–146
Barbosa LAG (1970) Carta Fitogeográfica de Angola. Instituto de Investigação Científica de Angola, Luanda
Blaine G (1922) Notes on the zebras and some antelopes of Angola. Proc Zool Soc London 92(2):317–339
Bocage JVB du (1890) Mammifères d’Angola et du Congo (Suite). Jornal de Sciencias, Mathemáticas, Physicas e Naturaes, Lisboa, Segunda Série 1(1):8–32
Crawford-Cabral J (1965) A palanca preta gigante, sua situação e medidas a adoptar. Luanda, Unpublished mimeograph
Crawford-Cabral J (1966) A palanca preta gigante, aditamentos e correcções ao relatório do ano anterior. Luanda, Unpublished mimeograph
Crawford-Cabral J (1969) A study of the giant sable. The zoological society of Southern Africa. News Bull 10(2):1–7
Crawford-Cabral J (1970) Alguns aspectos da ecologia da palanca real (Hippotragus niger variani Thomas). Boletim do Instituto de Investigação Científica de Angola 7:5–38
Crawford-Cabral J, Mesquitela LM (1989) Índice toponímico de colheitas zoológicas em Angola (Mammalia, Aves, Reptilia e Amphibia). Estudos, Ensaios e Documentos 151:1–206
Crawford-Cabral J, Veríssimo LN (2005) The ungulate fauna of Angola: systematic list, distribution maps, database report. Lisboa: Instituto de Investigação Científica Tropical, Lisboa
Curtis CP (1933) Giant sable antelope. In: Grinnell GB, Roosevelt K (eds) Hunting trails on three continents. Boone and Crocket Club, New York, pp 237–252
du Bocage JVB (1878) Liste des Antilopes d’Angola. Proc Zool Soc London 1878:741–745
Estes RD (1982) The giant sable and wildlife conservation in Angola. Report to IUCN/Species Survival Commission, Gland, Switzerland
Estes RD (2013) Hippotragus niger sable antelope. In: Kingdon J et al (eds) Mammals of Africa, vol 6. Bloomsbury, London, pp 556–565
Estes RD, Estes RK (1970) Preliminary report on the giant sable. Unpublished manuscript, p 22
Estes RD, Estes RK (1972) The giant sable antelope Hippotragus niger variani. Summary report and recommendations. Unpublished manuscript, p 55
Estes RD, Estes RK (1974) The biology and conservation of the giant sable antelope, Hippotragus niger variani Thomas, 1916. Proc Acad Natl Sci Phila 26:73–104
Frade F (1958) Mesure adoptées pour la protection de l’Hippotrague géant en Angola. Mammalia 22(3):476–477
Frade F (1967) Palanca Preta Gigante, Relíquia da Fauna de Angola. Unpublished mimeograph, p 3
Frade F, Sieiro D (1960) Palanca preta gigante de Angola. Garcia de Orta 8:21–38
Gray PN (1930) African game lands. The Sportsman 8(4):1–34
Gray PN (1933) Along the livingstone trail. In: Grinnell GB, Roosevelt K (eds) Hunting trails on three continents. Boone and Crocket Club, New York, pp 103–143
Groves C, Grubb P (2011) Ungulate taxonomy. Johns Hopkins University Press, Baltimore, p 317
Halse ARD (ed) (1998) Rowland Ward’s records of big game, XXV edn. Rowland Ward Publications, Johannesburg
Harper F (1945) Extinct and vanishing mammals of the old world. American Committee for International Wildlife Protection, New York
Heim F (1954) Les Fossiles des Demain: treize mammifères menaces d’extinction. Étudiés par le “Service de Sauvegarde”. Union Internationale pour la Protection de la Nature, p 112
Hill JE, Carter TD (1941) The mammals of Angola Africa. Bull Am Mus Nat Hist 78(1):1–211
Huntley BJ (1971) Guia Preliminar dos Parques e Reservas de Angola. Relatório 3. Repartição Técnica da Fauna, Direcção Provincial dos Serviços de Veterinária, Luanda, Mimeograph report, p 17
Huntley BJ (1972) Plano para o futuro da palanca real de Angola. Relatório 11. Repartição Técnica da Fauna, Direcção Provincial dos Serviços de Veterinária, Luanda, Mimeograph report, p 9
Huntley BJ (1973) Distribuição e situação da grande fauna selvagem da Angola com referência especial às espécies raras e em perigo de extinção – primeiro relatório sobre o estado actual. Relatório 21. Repartição Técnica da Fauna, Direcção Provincial dos Serviços de Veterinária, Luanda, Mimeograph report, p 14
Huntley BJ, Matos EM (1992) Biodiversity: Angolan environmental status quo assessment report. IUCN Regional Office for Southern Africa, Harare
IUCN SSC Antelope Specialist Group (2017) Hippotragus niger ssp. variani. The IUCN red list of threatened species 2017: e.T10169A50188611
Jansen van Vuuren BJ, Robinson TJ, Vaz Pinto P et al (2010) Western Zambian sable: are they a geographic extension of the giant sable antelope? S Afr J Wildl Res 40(1):35–42
Klein RG (1974) On the taxonomic status, distribution and ecology of the blue antelope, Hippotragus leucophaeus Pallas 1766. Ann S Afr Mus 65(4):99–143
Mathee CA, Robinson TJ (1999) Mitochondrial DNA population structure of roan and sable antelope: implications for the translocation and conservation of the species. Mol Ecol 8(2):227–238
Newton da Silva S (1958) A Caça e a Protecção da Fauna em Angola. Author’s Edition, Lisboa, p 174
Newton da Silva S (1970) A Grande Fauna Selvagem de Angola. Direcção Provincial dos Serviços de Veterinária, Luanda
Pedrosa V (1971) Deslocação à Reserva Natural e Integral do Luando. Direcção dos Serviços de Veterinária, Luanda, Unpublished manuscipt, p 24
Pitra C, Vaz Pinto P, O’Keeffe BW et al (2006) DNA-led rediscovery of the giant sable antelope in Angola. Eur J Wildl Res 52(3):145–152
Powell-Cotton PHG (1932) Angola. In: Maydon HC (ed) Big game shooting in Africa. Seeley, London, p 445
Schouteden H (1947) De zoogdieren van Belgisch-Congo en van Ruanda-Urundi (Les mammifères du Congo Belge et du Ruanda-Urundi), III. Ungulata (2) Rodentia. Annales du Musée du Congo Belge Série 2 3(3):333–576
Sieiro D (1962) Novas observações acerca da palanca preta gigante Ozanna grandicornis variani (Thomas). Boletim do Instituto de Investigação Científica de Angola 1:49–57
Silva JA (1972) A palanca real – contribuição para o estudo bioecológico da palanca real (Hippotragus niger variani). Junta de Investigações do Ultramar, Lisboa
Statham JCB (1922) Through Angola: a coming colony. Blackwood & Sons, London
Thomas O (1916) A new sable antelope from Angola. Proc Zool Soc Lond 1916:298–301
Varian HF (1953) Some African milestones. Books of Rhodesia, Bulawayo, p 272
Vaz Pinto P (2007) Hybridization in giant sable. A conservation crisis in a critically endangered Angolan icon. IUCN/SSC Antelope Spec Group Gnuslett 26:47–58
Vaz Pinto P (2018) Evolution history of the critically endangered giant sable antelope (Hippotragus niger variani) – insights into its phylogeography, population genetics, demography and conservation. PhD thesis. University of Porto, Porto
Vaz Pinto P, Lopes S, Mourão S et al (2015) First estimates of genetic diversity for the highly endangered giant sable antelope using a set of 57 microsatellites. Eur J Wildl Res 61(2):313–317
Vaz Pinto P, Beja P, Ferrand N et al (2016) Hybridization following population collapse in a critically endangered antelope. Sci Rep 6:18788
Walker JF (2004) A certain curve of horn: the hundred-year quest for the giant sable antelope of Angola. Grove/Atlantic Inc, New York
Wessels J (2007) Western Zambian sable: a giant sable look-alike or the real thing. Game Hunt 13:32–36
Wilson DE, Hirst SM (1977) Ecology and factors limiting roan and sable antelope populations in South Africa. Wildl Monogr 54:3–111
Zigelski P, Gomes A, Finckh M (2019) Suffrutex dominated ecosystems in Angola. In: Huntley BJ, Russo V, Lages F, Ferrand N (eds) Biodiversity of Angola. Science & conservation: a modern synthesis. Springer Nature, Cham
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Vaz Pinto, P. (2019). The Giant Sable Antelope: Angola’s National Icon. In: Huntley, B., Russo, V., Lages, F., Ferrand, N. (eds) Biodiversity of Angola. Springer, Cham. https://doi.org/10.1007/978-3-030-03083-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-03083-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03082-7
Online ISBN: 978-3-030-03083-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)