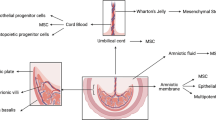
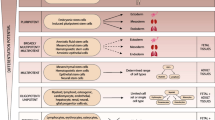
Abstract
Fetal stem/stromal cells can be isolated from various tissues and fluids including fetal blood, hematopoietic and somatic organs, amniotic fluid, and placenta. Fetal stem cells are emerging as a potential autologous and allogeneic cell source for several therapeutic applications in the field of cell therapy and regenerative medicine. While ageing of stem cells is not well understood, fetal stem/stromal cells are believed to have higher potential of growth and differentiation compared to their adult counterparts. The substantial advancements of cell banking have provided significant contribution to the rapid applications of cell therapy. Yet, the manufacturing of clinical grade fetal stem cells remains a hindrance due to the lack of a safe and effective cryopreservation compliant with good manufacturing practice for these cells. Whether fetal stem/stromal cells are to be used as an autologous or allogeneic cell therapy approach, therapeutic applications and outcomes are highly dependent on the long term ability to preserve these cells and maintain their viability and biological functions. The focus of this review is to discuss stem cells isolated from different fetal tissues and fluids, their cryopreservation approaches, and the commercial and regulatory aspects of these cells for therapeutic applications.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106(10):1613–23.
Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25(8):2017–24.
Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, et al. ‘Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res. 2007;313(12):2550–62.
Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13(2):219–24.
Poloni A, Maurizi G, Babini L, Serrani F, Berardinelli E, et al. Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant. 2011;20(5):643–54.
Lovelock JE. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953;11(1):28–36.
Woods EJ, Liu J, Zieger MA, Lakey JR, Critser JK. Water and cryoprotectant permeability characteristics of isolated human and canine pancreatic islets. Cell Transplant. 1999;8(5):549–59.
Woods EJ, Liu J, Gilmore JA, Reid TJ, Gao DY, et al. Determination of human platelet membrane permeability coefficients using the Kedem-Katchalsky formalism: estimates from two- vs three-parameter fits. Cryobiology. 1999;38(3):200–8.
Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48(2):146–56.
Eroglu A, Russo MJ, Bieganski R, Fowler A, Cheley S, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18(2):163–7.
Cox MA, Kastrup J, Hrubiško M. Historical perspectives and the future of adverse reactions associated with haemopoietic stem cells cryopreserved with dimethyl sulfoxide. Cell Tissue Bank. 2012;13(2):203–15.
Donmez A, Tombuloglu M, Gungor A, Soyer N, Saydam G, et al. Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci. 2007;36(1):95–101.
Syme R, Bewick M, Stewart D, Porter K, Chadderton T, et al. The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004;10(2):135–41.
Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, et al. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol Dis. 2009;34(1):1–10.
Aita K, Irie H, Tanuma Y, Toida S, Okuma Y, et al. Apoptosis in murine lymphoid organs following intraperitoneal administration of dimethyl sulfoxide (DMSO). Exp Mol Pathol. 2005;79(3):265–71.
Woods EJ, Pollok KE, Byers MA, Perry BC, Purtteman J, et al. Cord blood stem cell cryopreservation. Transfus Med Hemother. 2007;34(4):276–85.
Limaye LS. Bone marrow cryopreservation: improved recovery due to bioantioxidant additives in the freezing solution. Stem Cells. 1997;15(5):353–8.
Seo JM, Sohn MY, Suh JS, Atala A, Yoo JJ, et al. Cryopreservation of amniotic fluid-derived stem cells using natural cryoprotectants and low concentrations of dimethylsulfoxide. Cryobiology. 2011;62(3):167–73.
Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, et al. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004;13(3):295–305.
Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, et al. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56(2):144–51.
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61(10):3592–7.
Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek APM, et al. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem Toxicol. 2002;40(7):871–98.
Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36.
Erdag G, Eroglu A, Morgan J, Toner M. Cryopreservation of fetal skin is improved by extracellular trehalose. Cryobiology. 2002;44(3):218–28.
Beattie GM, Crowe JH, Lopez AD, Cirulli V, Ricordi C, et al. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. 1997;46(3):519–23.
Limaye LS, Kale VP. Cryopreservation of human hematopoietic cells with membrane stabilizers and bioantioxidants as additives in the conventional freezing medium. J Hematother Stem Cell Res. 2001;10(5):709–18.
Stolzing A, Naaldijk Y, Fedorova V, Sethe S. Hydroxyethylstarch in cryopreservation - mechanisms, benefits and problems. Transfus Apher Sci. 2012;46(2):137–47.
Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335(3):157–66.
Jones DR, Bui TH, Anderson EM, Ek S, Liu D, et al. In utero haematopoietic stem cell transplantation: current perspectives and future potential. Bone Marrow Transplant. 1996;18(5):831–7.
Bui TH, Jones DR. Stem cell transplantation into the fetal recipient: challenges and prospects. Curr Opin Obstet Gynecol. 1998;10(2):105–8.
Golfier F, Barcena A, Harrison MR, Muench MO. Fetal bone marrow as a source of stem cells for in utero or postnatal transplantation. Br J Haematol. 2000;109(1):173–81.
Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202.
Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013;9(2):226–40.
Ma L, Feng XY, Cui BL, Law F, Jiang XW, et al. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl). 2005;118(23):1987–93.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21(1):50–60.
Zhang YN, Lie PC, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy. 2009;11(5):548–58.
Zhao Q, Ren H, Li X, Chen Z, Zhang X, et al. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy. 2009;11(4):414–26.
Wu LF, Wang NN, Liu YS, Wei X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2009;15(10):2865–73.
Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37(5):629–40.
Wei X, Peng G, Zheng S, Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45(2):101–10.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259(2):150–6.
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–26.
van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp Hematol. 2007;35(12):1753–65.
Korbling M, Robinson S, Estrov Z, Champlin R, Shpall E. Umbilical cord blood-derived cells for tissue repair. Cytotherapy. 2005;7(3):258–61.
Lee MW, Choi J, Yang MS, Moon YJ, Park JS, et al. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Commun. 2004;320(1):273–8.
Lee MW, Yang MS, Park JS, Kim HC, Kim YJ, et al. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol. 2005;81(2):126–30.
Barachini S, Trombi L, Danti S, D’Alessandro D, Battolla B, et al. Morpho-functional characterization of human mesenchymal stem cells from umbilical cord blood for potential uses in regenerative medicine. Stem Cells Dev. 2009;18(2):293–305.
Kedong S, Xiubo F, Tianqing L, Macedo HM, LiLi J, et al. Simultaneous expansion and harvest of hematopoietic stem cells and mesenchymal stem cells derived from umbilical cord blood. J Mater Sci Mater Med. 2010;21(12):3183–93.
Phuc PV, Nhung TH, Loan DT, Chung DC, Ngoc PK. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In Vitro Cell Dev Biol Anim. 2011;47(1):54–63.
Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, et al. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30(7):569–75.
Zhang HT, Cheng HY, Zhang L, Fan J, Chen YZ, et al. Umbilical cord blood cell-derived neurospheres differentiate into Schwann-like cells. Neuroreport. 2009;20(4):354–9.
Luo YC, Zhang HT, Cheng HY, Yang ZJ, Dai YW, et al. Differentiation of cryopreserved human umbilical cord blood-derived stromal cells into cells with an oligodendrocyte phenotype. In Vitro Cell Dev Biol Anim. 2010;46(7):585–9.
Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, et al. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16(6):579–85.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301.
Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233(7):901–13.
Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, et al. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int J Hematol. 2009;90(2):261–9.
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402.
In ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van C, der Keur C, Kruisselbrink AB, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–52.
Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25(3):646–54.
Brady K, Dickinson SC, Guillot PV, Polak J, Blom AW, et al. Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis. Stem Cells Dev. 2014;23(5):541–54.
Weber B, Zeisberger SM, Hoerstrup SP. Prenatally harvested cells for cardiovascular tissue engineering: fabrication of autologous implants prior to birth. Placenta. 2011;32(4):14.
Delo DM, De Coppi P, Bartsch Jr G, Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426–38.
Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, et al. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196(4):592–7.
Joo S, Ko IK, Atala A, Yoo JJ, Lee SJ. Amniotic fluid-derived stem cells in regenerative medicine research. Arch Pharm Res. 2012;35(2):271–80.
Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, et al. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg. 2007;42(6):974–9.
Hosper NA, Bank RA, van den Berg PP. Human Amniotic Fluid-Derived Mesenchymal Cells from Fetuses with a Neural Tube Defect Do Not Deposit Collagen Type I Protein After TGF-beta1 Stimulation In Vitro. Stem Cells Dev. 2014;23(5):555–62.
Di Bernardo J, Maiden MM, Hershenson MB, Kunisaki SM. Amniotic fluid derived mesenchymal stromal cells augment fetal lung growth in a nitrofen explant model. J Pediatr Surg. 2014;49(6):859–64.
Moschidou D, Mukherjee S, Blundell MP, Jones GN, Atala AJ, et al. Human mid-trimester amniotic fluid stem cells cultured under embryonic stem cell conditions with valproic acid acquire pluripotent characteristics. Stem Cells Dev. 2013;22(3):444–58.
Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16(6):931–52.
Lai D, Wang F, Chen Y, Wang L, Wang Y, et al. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Dev Biol. 2013;13:34.
Yu X, Wang N, Qiang R, Wan Q, Qin M, et al. Human amniotic fluid stem cells possess the potential to differentiate into primordial follicle oocytes in vitro. Biol Reprod. 2014;90:73.
Kmiecik G, Niklinska W, Kuc P, Pancewicz-Wojtkiewicz J, Fil D, et al. Fetal membranes as a source of stem cells. Adv Med Sci. 2013;58(2):185–95.
Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30(1):2–10.
Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):877–91.
Bacenkova D, Rosocha J, Tothova T, Rosocha L, Sarissky M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy. 2011;13(9):1047–56.
Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9(1):16–31.
Castrechini NM, Murthi P, Gude NM, Erwich JJ, Gronthos S, et al. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31(3):203–12.
Castrechini NM, Murthi P, Qin S, Kusuma GD, Wilton L, et al. Decidua parietalis-derived mesenchymal stromal cells reside in a vascular niche within the choriodecidua. Reprod Sci. 2012;19(12):1302–14.
Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17(8):955–68.
Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78(10):1439–48.
Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, et al. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18(4):405–22.
Miki T, Mitamura K, Ross MA, Stolz DB, Strom SC. Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol. 2007;75(2):91–6.
Zhang Y, Li CD, Jiang XX, Li HL, Tang PH, et al. Comparison of mesenchymal stem cells from human placenta and bone marrow. Chin Med J (Engl). 2004;117(6):882–7.
Fazekasova H, Lechler R, Langford K, Lombardi G. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng Regen Med. 2011;5(9):684–94.
Li C, Zhang W, Jiang X, Mao N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007;330(3):437–46.
Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15(7):539–47.
Zhang Y, Li C, Jiang X, Zhang S, Wu Y, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32(7):657–64.
Lee HJ, Jung J, Cho KJ, Lee CK, Hwang SG, et al. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation. 2012;84(3):223–31.
Iwasaki R, Matsubara S, Takizawa T, Takayama T, Yashiro T, et al. Human amniotic epithelial cells are morphologically homogeneous: enzyme histochemical, tracer, and freeze-substitution fixation study. Eur J Histochem. 2003;47(3):223–32.
Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, Rendal-Vazquez ME, Fuentes-Boquete I, et al. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J Cell Biochem. 2010;111(4):846–57.
Ochsenbein-Kolble N, Bilic G, Hall H, Huch R, Zimmermann R. Inducing proliferation of human amnion epithelial and mesenchymal cells for prospective engineering of membrane repair. J Perinat Med. 2003;31(4):287–94.
Miki T, Marongiu F, Ellis EC, Dorko K, Mitamura K, et al. Production of hepatocyte-like cells from human amnion. Methods Mol Biol. 2009;481:155–68.
Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–88.
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–59.
Pozzobon M, Piccoli M, De Coppi P. Stem cells from fetal membranes and amniotic fluid: markers for cell isolation and therapy. Cell Tissue Bank. 2014;15(2):199–211.
Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–7.
Zhao H-X, Li Y, Jin H-F, Xie L, Liu C, et al. Rapid and efficient reprogramming of human amnion-derived cells into pluripotency by three factors OCT4/SOX2/NANOG. Differentiation. 2010;80(2–3):123–9.
Hou Y, Huang Q, Liu T, Guo L. Human amnion epithelial cells can be induced to differentiate into functional insulin-producing cells. Acta Biochim Biophys Sin (Shanghai). 2008;40(9):830–9.
Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res Ther. 2013;4(5):124.
Smagur A, Mitrus I, Giebel S, Sadus-Wojciechowska M, Najda J, et al. Impact of different dimethyl sulphoxide concentrations on cell recovery, viability and clonogenic potential of cryopreserved peripheral blood hematopoietic stem and progenitor cells. Vox Sang. 2013;104(3):240–7.
Mitrus I, Smagur A, Giebel S, Gliwinska J, Prokop M, et al. A faster reconstitution of hematopoiesis after autologous transplantation of hematopoietic cells cryopreserved in 7.5 % dimethyl sulfoxide if compared to 10 % dimethyl sulfoxide containing medium. Cryobiology. 2013;67(3):327–31.
Abrahamsen JF, Bakken AM, Bruserud O. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion. 2002;42(12):1573–80.
Galmes A, Besalduch J, Bargay J, Novo A, Morey M, et al. Long-term storage at -80 degrees C of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide as the sole cryoprotectant. Transfusion. 1999;39(1):70–3.
Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, et al. Uncontrolled-rate freezing and storage at -80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41(5):667–73.
Son JH, Heo YJ, Park MY, Kim HH, Lee KS. Optimization of cryopreservation condition for hematopoietic stem cells from umbilical cord blood. Cryobiology. 2010;60(3):287–92.
Rowley SD, Anderson GL. Effect of DMSO exposure without cryopreservation on hematopoietic progenitor cells. Bone Marrow Transplant. 1993;11(5):389–93.
Branch DR, Calderwood S, Cecutti MA, Herst R, Solh H. Hematopoietic progenitor cells are resistant to dimethyl sulfoxide toxicity. Transfusion. 1994;34(10):887–90.
Motta JPR, Gomes BE, Bouzas LF, Paraguassú-Braga FH, Porto LC. Evaluations of bioantioxidants in cryopreservation of umbilical cord blood using natural cryoprotectants and low concentrations of dimethylsulfoxide. Cryobiology. 2010;60(3):301–7.
Sasnoor LM, Kale VP, Limaye LS. Supplementation of conventional freezing medium with a combination of catalase and trehalose results in better protection of surface molecules and functionality of hematopoietic cells. J Hematother Stem Cell Res. 2003;12(5):553–64.
Sasnoor LM, Kale VP, Limaye LS. A combination of catalase and trehalose as additives to conventional freezing medium results in improved cryoprotection of human hematopoietic cells with reference to in vitro migration and adhesion properties. Transfusion. 2005;45(4):622–33.
Meyer TP, Hofmann B, Zaisserer J, Jacobs VR, Fuchs B, et al. Analysis and cryopreservation of hematopoietic stem and progenitor cells from umbilical cord blood. Cytotherapy. 2006;8(3):265–76.
Hunt CJ, Armitage SE, Pegg DE. Cryopreservation of umbilical cord blood: 2. Tolerance of CD34+ cells to multimolar dimethyl sulphoxide and the effect of cooling rate on recovery after freezing and thawing. Cryobiology. 2003;46(1):76–87.
Solves P, Mirabet V, Planelles D, Carbonell-Uberos F, Roig R. Influence of volume reduction and cryopreservation methodologies on quality of thawed umbilical cord blood units for transplantation. Cryobiology. 2008;56(2):152–8.
Naaldijk Y, Staude M, Fedorova V, Stolzing A. Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide. BMC Biotechnol. 2012;12:49.
Almici C, Carlo-Stella C, Wagner JE, Mangoni L, Garau D, et al. Clonogenic capacity and ex vivo expansion potential of umbilical cord blood progenitor cells are not impaired by cryopreservation. Bone Marrow Transplant. 1997;19(11):1079–84.
Guttridge MG, Soh TG, Belfield H, Sidders C, Watt SM. Storage time affects umbilical cord blood viability. Transfusion. 2014;54:1278.
Pereira-Cunha FG, Duarte AS, Costa FF, Saad ST, Lorand-Metze I, et al. Viability of umbilical cord blood mononuclear cell subsets until 96 hours after collection. Transfusion. 2013;53(9):2034–42.
Wang HY, Lun ZR, Lu SS. Cryopreservation of umbilical cord blood-derived mesenchymal stem cells without dimethyl sulfoxide. Cryo Letters. 2011;32(1):81–8.
Balci D, Can A. The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr Stem Cell Res Ther. 2013;8(1):60–72.
Gong W, Han Z, Zhao H, Wang Y, Wang J, et al. Banking human umbilical cord-derived mesenchymal stromal cells for clinical use. Cell Transplant. 2012;21(1):207–16.
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–11.
Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5(3):143–54.
Thirumala S, Goebel WS, Woods EJ. Manufacturing and banking of mesenchymal stem cells. Expert Opin Biol Ther. 2013;13(5):673–91.
Miranda-Sayago JM, Fernandez-Arcas N, Benito C, Reyes-Engel A, Herrero JR, et al. Evaluation of a low cost cryopreservation system on the biology of human amniotic fluid-derived mesenchymal stromal cells. Cryobiology. 2012;64(3):160–6.
Cho HJ, Lee SH, Yoo JJ, Shon YH. Evaluation of cell viability and apoptosis in human amniotic fluid-derived stem cells with natural cryoprotectants. Cryobiology. 2014;68(2):244–50.
Heng BC, Clement MV, Cao T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Biosci Rep. 2007;27(4-5):257–64.
Montolio M, Tellez N, Biarnes M, Soler J, Montanya E. Short-term culture with the caspase inhibitor z-VAD.fmk reduces beta cell apoptosis in transplanted islets and improves the metabolic outcome of the graft. Cell Transplant. 2005;14(1):59–65.
Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, et al. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas. 2004;29(2):104–9.
Janz Fde L, Debes Ade A, Cavaglieri Rde C, Duarte SA, Romao CM, et al. Evaluation of distinct freezing methods and cryoprotectants for human amniotic fluid stem cells cryopreservation. J Biomed Biotechnol. 2012;2012:649353.
Steigman SA, Armant M, Bayer-Zwirello L, Kao GS, Silberstein L, et al. Preclinical regulatory validation of a 3-stage amniotic mesenchymal stem cell manufacturing protocol. J Pediatr Surg. 2008;43(6):1164–9.
Ng SC, Sathananthan H, Bongso A, Lee MN, Mok H, et al. The use of amniotic fluid and serum with propanediol in freezing of murine 2-cell embryos. Fertil Steril. 1988;50(3):510–3.
Dorfmann AD, Bender SD, Robinson P, Fugger EF, Bustillo M, et al. Cell-free human amniotic fluid as culture medium for mouse and human embryos. Fertil Steril. 1989;51(4):671–4.
Niknejad H, Deihim T, Peirovi H, Abolghasemi H. Serum-free cryopreservation of human amniotic epithelial cells before and after isolation from their natural scaffold. Cryobiology. 2013;67(1):56–63.
Murphy S, Rosli S, Acharya R, Mathias L, Lim R et al. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol 2010; Chapter 1: Unit 1E.6.
Zarzeczny A, Rachul C, Nisbet M, Caulfield T. Stem cell clinics in the news. Nat Biotechnol. 2010;28(12):1243–6. doi:10.1038/nbt1210-1243b.
Regenberg AC, Hutchinson LA, Schanker B, Mathews DJ. Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells. 2009;27(9):2312–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Albanna, M.Z., Woods, E.J. (2016). Fetal Stem Cell Banking. In: Fauza, D., Bani, M. (eds) Fetal Stem Cells in Regenerative Medicine. Stem Cell Biology and Regenerative Medicine. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3483-6_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3483-6_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3481-2
Online ISBN: 978-1-4939-3483-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)