Abstract
Airborne pathogens entering the lungs first encounter the mucus layer overlaying epithelial cells as a first line of host defense [1, 2]. In addition to serving as the physical barrier to these toxic agents, intact epithelia also are major sources of various macromolecules including antimicrobial agents, antioxidants and antiproteases [3, 4] as well as proinflammatory cytokines and chemokines that initiate and amplify host defensive responses to these toxic agents [5]. Airway epithelial cells can be categorized as either ciliated or secretory [6]. Secretory cells, such as goblet cells and Clara cells, are responsible for the production and secretion of mucus along the apical epithelial surface and, in conjunction with ciliated cells, for the regulation of airway surface liquid viscosity. In addition, submucosal mucus glands connect to the airway lumen through a ciliated duct that propels mucins outward. These glands are present in the larger airways between bands of smooth muscle and cartilage. See Fig. 1.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Airway Inflammation
- Airway Epithelial Cell
- Neutrophil Elastase
- MUC1 Expression
- Normal Human Bronchial Epithelial
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Airborne pathogens entering the lungs first encounter the mucus layer overlaying epithelial cells as a first line of host defense [1, 2]. In addition to serving as the physical barrier to these toxic agents, intact epithelia also are major sources of various macromolecules including antimicrobial agents, antioxidants and antiproteases [3, 4] as well as proinflammatory cytokines and chemokines that initiate and amplify host defensive responses to these toxic agents [5]. Airway epithelial cells can be categorized as either ciliated or secretory [6]. Secretory cells, such as goblet cells and Clara cells, are responsible for the production and secretion of mucus along the apical epithelial surface and, in conjunction with ciliated cells, for the regulation of airway surface liquid viscosity. In addition, submucosal mucus glands connect to the airway lumen through a ciliated duct that propels mucins outward. These glands are present in the larger airways between bands of smooth muscle and cartilage. See Fig. 1.
Initially, inhaled toxic agents encounter a mucus layer overlying the respiratory epithelium, become trapped, and are subsequently neutralized by macromolecules. Elimination of these toxic agents depends on mucociliary clearance and cough. Continuous ciliary movement propels secretions proximally at about 1 mm per minute [7], and this mucosal velocity is modified by hydration of the mucus layer [7, 8] and adrenergic and cholinergic stimuli [7, 9–11]. Efficacy of cough in the elimination of mucus depends on inspiratory muscle strength and expiratory flow velocity, which must detach the mucus from the airway surface and expel the secretions proximally.
A second layer of defense is provided by cell surface receptors (e.g. Toll-like receptors, TLRs) on epithelial cells and resident leukocytes. They bind to various components of the harmful agents and stimulate the production of proinflammatory cytokines (e.g. tumor necrosis factor-α, TNF-α) and chemokines (e.g. interleukin-8). Finally, a third layer of protection is mediated by leukocytes recruited to the lumen of the airways by chemotactic molecules that attack pathogens by direct phagocytosis, as well as through the release anti-microbial proteases (e.g. neutrophil elastase) and oxygen radicals. Details of the role of airway epithelium in the host immune responses are described in a recent review [12]. This review is focused on the role of airway mucus, particularly MUC1 mucin, in the context of chronic inflammatory lung diseases.
Clinical Consequences and Airway Pathology
Mucus is overproduced in several inflammatory lung diseases, a process detrimental to the normal lung defense against environmental toxins. To make matters worse, difficulty in secretion clearance secondary to ineffective cough or poor mucociliary transport leads to mucostasis and paradoxically predisposes to bacterial colonization and infection [13, 14]. Dyspnea, cough and sputum production result from the physical obstruction of the airways by mucus and stimulation of intrapulmonary vagal afferent nerves [15, 16]. Lung diseases such as COPD, bronchiectasis, and cystic fibrosis are characterized by mucus hypersecretion, chronic bacterial colonization, and repeated lower respiratory tract infections [17–21].
Airway disease is a crucial pathologic component of multiple inflammatory lung diseases (See Table 1). Pathologic changes in airway epithelium of COPD patients include squamous metaplasia, inflammatory cell infiltration, goblet cell hyperplasia, and mucus metaplasia, a process in which mucus is overproduced in response to inflammatory stimuli (Figs. 2 and 3) [22]. These abnormalities are seen in both the larger central airways as well as smaller respiratory bronchioles [23–26]. Airway inflammation from smoking begins early in the course of the disease, and leads to persistent and progressive airway remodeling, even after smoking cessation [27]. Niewoehner et al. discovered inflammatory changes in the peripheral airways of young smokers who died suddenly, suggesting that airway disease developed before the diagnosis of COPD could be established [28]. As further evidence of this concept, epithelial layer thickness and mucous metaplasia increase incrementally with disease severity [26, 29]. These alterations in the epithelium increase airflow obstruction by several mechanisms: (1) excess mucus occludes the airway lumen [30]; (2) epithelial layer thickening encroaches on the airway lumen, thereby reducing inner diameter [31]; and (3) increased mucus alters surface tension of the airway, predisposing it to collapse during expiration [32]. Hogg et al. found inverse relationships between inflammatory cell infiltration and luminal occlusion of the small airways and lung function [29], strongly supporting the notion that small airway pathology is responsible for severity of illness. Airway disease also has prognostic significance in COPD. Mucus metaplasia in COPD has been associated with worse physiologic response to lung volume reduction surgery [33] as well as greater mortality [34].
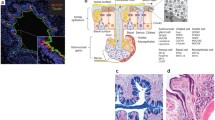
Examples of airway remodeling in COPD. A represents mucous metaplasia (MM) of the epithelium and smooth muscle hypertrophy (SM). B represents peribronchial fibrosis (black arrow). C shows squamous metaplasia. D shows an inflammatory infiltrate of lymphocytes in the adventitia of a bronchiole (Reprinted with permission from the American Thoracic Society. Copyright© American Thoracic Society. Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thor Soc 2008; 5: 478–485. Official Journal of the American Thoracic Society)
Periodic Acid Fluorescent Schiff stain of a small airway from a patient with advanced emphysema. The entire airway is seen in A and a quadrant of the airway in B. Mucin granules are shown in red along the apical border of the epithelium. Note the large intralumenal mucin plug (M) in A, also noted in B (white arrow) (Reprinted with permission from the American Thoracic Society. Copyright© American Thoracic Society. Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thor Soc 2008; 5: 478–485. Official Journal of the American Thoracic Society)
Although it is established that quantity of emphysema correlates well with clinical disease staging in COPD, the relationship between airway pathology, physiology and symptom severity is weak at best. Chronic bronchitis exists in 26–45 % of all smokers, but COPD develops in only 15–20 % [35, 36]. Large airway mucous metaplasia correlates poorly with the degree of airflow obstruction[37] and amount of mucus expectoration [38]. Small airway disease has been found in surgical lung specimens from those with advanced emphysema, with no clinical or radiographic evidence to suggest its presence preoperatively [29, 33, 39]. More importantly, the degree of small airway mucous metaplasia is difficult to detect clinically by burden of cough or sputum [40].
Despite the disconnect between symptoms and airway pathology, chronic cough and sputum production in COPD have multiple consequences, including an accelerated decline in lung function, [41, 42] increased exacerbation frequency [43–47], greater respiratory symptoms [43, 48], worse health related quality of life [43], and higher mortality [35, 49]. These phenomena are without a doubt a result of increased airway inflammation and worsened airflow obstruction, in addition to the aforementioned mechanisms. In a long term study of over 9,000 adults, an excess yearly rate of FEV1 decline of 12.6–22.8 mL was attributed to chronic mucus hypersecretion [21]. We have found chronic cough and sputum production in patients with severe COPD were associated with higher dyspnea scores and more upper airway symptoms [43, 48]. In multiple studies, patients with chronic bronchitis and COPD were found to be at a 1.20–1.92-fold increased risk for COPD exacerbation compared to those without chronic bronchitis [43–47]. The cause of the observed increase in all-cause mortality, however, is still a matter of debate. It is hypothesized that the increased lung inflammation associated with chronic bronchitis causes greater systemic inflammation, resulting in numerous downstream consequences, including coronary artery disease, dyslipidemia, osteoporosis, and skeletal muscle weakness [50]. In the Tucson Epidemiological Survey of Airway Obstructive Disease, chronic bronchitis was associated with a 2.2-fold greater risk of all-cause mortality in those under the age of 50, and was also associated with higher serum levels of IL-8 and C-reactive protein at enrollment [49].
In asthma, chronic inflammation and thickening of the small airway epithelium, submucosal space, and smooth muscle has been noted in several pathologic studies [51–53]. In addition, shedding of the epithelial layer has been noted in postmortem studies, bronchoalveolar lavage fluid, and sputum samples [54], most likely as a result of weakened attachment of epithelial cells to the basement membrane. Large airway goblet cell hyperplasia and smooth muscle hypertrophy are prominent pathologic features of asthma. Goblet cell hyperplasia is more consistently seen in asthma compared to COPD, where clinical and pathologic phenotype is a highly variable combination of airway disease and emphysema. In asthma, mucus hypersecretion leads to obstruction of the majority of distal airways and ultimately respiratory failure during fatal asthma exacerbations [55]. Diffuse occlusion of the small and medium sized airways by mucus and cellular debris has been demonstrated in multiple autopsy studies of patients who died from asthma [56, 57]. Goblet cell hyperplasia is also seen in less severe cases as well; Ordonez et al. found a greater number of goblet cells and secreted mucins in subjects with mild to moderate asthma compared to control subjects [58].
Similar to asthma, mucus hypersecretion in cystic fibrosis leads to airflow obstruction and small airway occlusion [59]. However, cystic fibrosis is caused by dysfunction of an epithelial chloride channel, which results in sodium and water influx to the epithelial layer and therefore depletion of the airway surface liquid [19]. The increased viscosity and tenacity of secretions makes detachment from the epithelium and propulsion outward during cough exceedingly difficult. Excess mucus production combined with airway surface liquid dessication results in mucostasis, causing colonization by pathogenic bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia [18–20].
Airway Mucus and Mucins
Mucus, or the airway surface liquid, is a complex mixture of ions, salts, peptides, proteins, glycoconjugates and water. Strict regulation of mucus production is indispensable for normal lung function. The protective function of mucus depends on its proper composition of constituent components, particularly mucin glycoproteins. Mucins are high molecular weight proteins with O-glycosidic linkages between the first GalNAc residue of the oligosaccharide side chains and serine and threonine amino acids of the polypeptide backbone. Over 20 mucin (MUC in human, Muc in animals) genes have been cloned, 12 of which are expressed in the lung [2, 60]. The airway mucin include secreted gene products (MUC2, 5AC, 5B, 7, 8, and 19) and membrane-tethered mucins (MUC1, 4, 11, 13, 16, and 20). MUC5AC and MUC5B are the two major secretory mucins in the respiratory tract. The levels of these mucins in mucus have been shown to significantly increase, and to directly correlate with, the number of goblet cells under the pathological conditions of goblet cell metaplasia or goblet cell hyperplasia. Although the exact roles of MUC5AC and MUC5B in the airways remain to be fully elucidated, it has been suggested that MUC5AC expression is inducible during airway inflammation, whereas MUC5B expression is constitutive [61]. A recent report supports this notion by demonstrating that MUC5AC levels correlated with the degree of airway obstruction in COPD patients [62]. Cystic fibrosis, in contrast, is characterized by greater MUC5B levels compared to MUC5AC [63, 64], suggesting that impaired mucociliary clearance is the principal mechanism responsible for the overwhelming burden of mucus in these patients.
Epithelial TLRs as Mediators of Airway Inflammation
TLRs, and related molecules, on airway epithelial cells comprise a second line of defense against inhaled microbial pathogens [65]. These pattern recognition receptors (PRRs) constitute an evolutionary conserved family of gene products that interact with pathogen-associated molecular patterns (PAMPs) to initiate downstream signal transduction and innate inflammatory responses. In general, all TLRs possess a leucine-rich repeat region in their ectodomains and an intracellular Toll/interleukin-1 receptor (TIR) domain. TLR signaling is activated by agonist-induced receptor homodimerization, recruitment of cytoplasmic adaptor proteins (MyD88, TIRAP, TRIF) to the TIR domain, and activation of protein kinases (IRAKs, TRAF6) [66]. Although all of the 10 known human TLRs are expressed by airway epithelial cells, TLR2 and TLR5 are the predominant respiratory PRRs [67, 68]. TLR5 engages flagellin, the major protein component of the bacterial flagellum, while TLR2 recognizes a diverse array of components from Gram-positive and Gram-negative bacteria, including lipoproteins and peptidoglycan. It remains unclear how a single receptor (TLR2) can recognize such a broad diversity of stimuli, but a possible explanation is the ability of TLR2 to form heterodimers with TLR1 and TLR6. For example, bacterial peptidoglycan interacted with the TLR2/6 co-receptor complex on airway epithelial cells to activate NF-κB and stimulate production of TNF-α [69]. The magnitude of the response generated by the TLR2/6 heterodimer was greater than that produced by TLR2 alone. While TLR5 homodimers are clearly capable of binding to flagellin, Mizel et al. [70] reported that nitric oxide production by airway epithelial cells in response to flagellin was dependent on interaction of TLR5 with TLR4. TLR2 also was shown to be involved in signaling induced by flagellin in human airway epithelial cells, suggesting a possible TLR2/TLR5 heterodimer interaction [67]. Biotinylation of surface proteins of airway epithelial cells followed by co-immunoprecipitation experiments demonstrated that both TLR2 and TLR5 were associated with the ganglioside, asialoGM1, in the plasma membrane [71]. IRAK1 and TRAF6 were also found in the co-receptor complex, whereas TLR4 was not. Furthermore, treatment of airway epithelial cells with Pseudomonas aeruginosa pili or flagella mobilized asialoGM1, TLR2, and TLR5 to the apical surface of the cells leading to Ca+2-associated activation of mitogen-activated protein kinases (MAPKs), nuclear translocation of NF-κB, and production of IL-8 [67]. These combined results indicate that TLRs link the asialoGM1 glycoconjugate to intracellular signal transduction leading to a proinflammatory host response following interaction with bacterial components. Other groups, however, have questioned the role of asialoGM1 as a cell surface receptor for P. aeruginosa, particularly clinical isolates of the bacterium [72].
Neutrophil Elastase in the Airways
Neutrophils and macrophages constitute a third layer of defense in the clearance of bacteria from the lungs. The anti-microbial function of these immune cells is directly mediated through phagocytosis, and indirectly by the release of anti-microbial agents [73]. Among the soluble mediators released by neutrophils is the serine protease, neutrophil elastase (NE). Studies using NE knockout mice showed that this protease is required for host defense against experimental infection by Gram-negative bacteria [74]. However, the role of NE in the normal lung response to spontaneous bacterial infection needs to be more firmly established. Some evidence suggests that NE promotes neutrophil migration into the lung by degradation of the extracellular matrix, but this remains controversial [75]. In general, NE is considered as a proinflammatory molecule, and NE chemical inhibitors decrease inflammation and lung edema in animal models [76]. Part of the mechanism through which NE mediates its anti-microbial effects is up-regulation of mucin secretion by goblet cells [77]. Using a co-culture system containing neutrophils and primary tracheal epithelial cells, Kim et al. [78] demonstrated that activation by fMLP/cytochalasin B resulted not only in increased NE production by neutrophils, but also greater mucin release from the epithelial cells. Both effects were blocked in a dose-dependent fashion by pretreatment with α1-protease inhibitor, implicating a proteolytic effect of NE on the epithelial cells. Kohri et al. [79] reported that NE treatment of NCI-H292 airway epithelial cells stimulated the production of MUC5AC mucin through transforming growth factor-α (TGF-α)-dependent activation of the epidermal growth factor receptor (EGFR). Park et al. [80] showed that NE treatment of well-differentiated primary normal human bronchial epithelial (NHBE) cells cultured at an air-liquid interface (ALI) increased the release of MUC5AC and MUC5B mucins via an intracellular signaling pathway involving protein kinase Cδ (PKCδ). To date, NE is the most potent mucin secretagogue described.
Control of Airway Inflammation
Given the intricate and diverse host airway inflammatory mechanisms, a critical balance between these processes and the counter-regulating anti-inflammatory pathways is absolutely required to maintain a homeostatic environment in the airways. This balance ensures that harmful environmental insults are effectively neutralized without excessive bystander tissue damage. Although a large body of literature has characterized the microbial-stimulated pro-inflammatory pathways summarized above, relatively less is known about the compensatory anti-inflammatory responses. Nevertheless, it is hypothesized that failure to down-regulate airway inflammation results in the development of acute or chronic respiratory diseases, including COPD, CF, ARDS, and asthma [25]. A number of anti-inflammatory molecules have been shown to play an important role in controlling the normal inflammatory response in the lung, including IL-10, transforming growth factor-β (TGF-β), peroxisome proliferator activating receptor (PPAR)-γ, and Mucin-1 (MUC1) [25, 81, 82]. However, what is less clear is whether defective expression and/or structure/function of these, or related, anti-inflammatory mediators is responsible for the etiopathogenesis of inflammatory lung diseases. The following sections briefly describe each of these key anti-inflammatory mediators with the goal of stimulating further basic and clinical research on their role in airway inflammatory diseases.
Interleukin-10
IL-10 down-regulates the expression of proinflammatory cytokines, including interferon-γ (IFN-γ), IL-2, and TNF-α, major histocompatibility complex (MHC) class II antigens, and leukocyte co-stimulatory molecules [83]. IL-10 also enhances B cell survival, proliferation, and antibody production. These pleiotropic effects are mediated through interaction of the IL-10 homodimer with its cognate IL-10 receptor α subunit (IL-10Rα), and subsequent binding of this ligand-receptor complex to the IL-10R2 co-receptor. An accumulating body of evidence points toward a role for IL-10 in chronic inflammation during COPD and asthma [84, 85], although a direct causal effect for IL-10 in the pathogenesis of these disorders is unclear. In the case of CF, airway secretions from afflicted patients, as well as CFTR−/− mice, have decreased IL-10 levels compared with secretions from normal individuals or CFTR+/+ mice [82].
Transforming Growth Factor-β
TGF-β is an anti-inflammatory cytokine that exists in three isoforms, TGF-β1, -β2 and -β3. TGF-β knockout mice are embryonic lethal as a result of profound multiorgan inflammation. TGF-β+/− heterozygous mice have reduced levels of the cytokine and exhibit exacerbated airway inflammation compared with wild type animals, suggesting a role for endogenous TGF-β in suppressing the development of allergic airway disease [86]. Additional evidence supporting an anti-inflammatory role for TGF-β comes from the observation that intratracheal delivery of TGF-β suppressed allergen-induced airway inflammation in a murine model of asthma [87]. Increased airway inflammation also was evident upon inhibition of TGF-β-dependent intracellular signaling [88]. Genetic studies have demonstrated an association between gene polymorphisms of the TGF-β locus and COPD [89]. Finally, a possible role for TGF-β in CF comes from the report that CF human cell lines and cells from CFTR−/− mice have decreased Smad3 levels and decreased responses to TGF-β [90].
Peroxisome Proliferator Activating Receptor-γ
PPAR-α, -β, and -γ are members of the steroid hormone receptor family of ligand-activated transcription factors [82]. PPARs form heterodimers with retinoid X receptors that regulate gene transcription. PPARγ is expressed as two isoforms, PPARγ1 and PPARγ2, that differ by the presence of a unique 30 amino acid segment in the latter [91]. PPARγ2 is primarily expressed in adipose tissue, while PPARγ1 is expressed in the lung, heart, skeletal muscle, intestine, kidney, pancreas, spleen, breast, and lymphoid tissues [92]. Both PPARγ molecules are activated by prostanoids, a subclass of eicosanoids consisting of prostaglandins, thromboxanes, and prostacyclins. Synthetic PPARγ ligands, such as the thiazolidinediones [93], have been developed that suppress inflammation both in vitro and in vivo [94, 95], including in response to lung infection with Pseudomonas aeruginosa [96], the major bacterial species that is responsible for the morbidity and mortality of CF. In the case of CF, at least three lines of evidence have been reported for an anti-inflammatory role for PPAR-γ. First, PPAR-γ inhibits airway inflammation by competitively inhibiting NF-κB binding to gene promoters, thereby blocking the activation of proinflammatory cytokines [97]. Second, PPAR-γ expression is decreased in lung of CFTR−/− mice compared with CFTR+/+ mice [98]. Finally, CF airway epithelial cell lines have reduced PPAR-γ levels compared with normal cells [99]. Thus, decreased PPAR-γ expression likely contributes to defective NF-κB signaling that favors increased airway inflammation in CF, and possibly other inflammatory airway diseases. However, the exact mechanisms by which PPARγ down-regulates inflammatory responses in CF and other lung diseases remain to be clarified.
MUC1 Mucin
Of the 20 known mucin genes, MUC1 was the first to be cloned [100, 101]. MUC1 is a single pass, transmembrane glycoprotein located on the apical surface of airway epithelial cells and is composed of two polypeptide chains, a large molecular weight (>250 kDa) subunit containing glycosylated variable number of tandem repeats (VNTR) and a SEA (sea urchin sperm protein, enterokinase, agrin) domain, and a 25 kDa subunit comprised of the transmembrane and intracellular COOH-terminus (CT) regions of the molecule [25]. The two polypeptide structure of MUC1 arises as a consequence of proteolysis within the SEA domain [102]. MUC1 is unique among the membrane-bound mucins because its CT region constitutes a signal transduction domain. The CT contains multiple amino acid sequence motifs predicted as binding sites for Shc, c-Src, Grb-2, β-catenin, and phosphoinositide 3-kinase (PI3K) [25]. These motifs are evolutionarily conserved and undergo tyrosine phosphorylation. The presence of CT phosphorylation sites associated with signaling cascades that have been characterized for other membrane receptors has suggested that MUC1 is functionally analogous to cytokine and growth factor receptors [103].
Anti-Inflammatory Role of MUC1 in Airway Epithelia
Identification of a functional role for MUC1 in the airways was made possible by the generation of Muc1 knockout (Muc1−/−) mice [104]. Early experiments demonstrated that Muc1−/− mice were predisposed to developing spontaneous eye inflammation due to infections by Staphylococcus, Streptococcus, or Corynebacterium compared with wild type animals with an intact Muc1 gene [105]. Subsequent studies by Lu et al. [106] using an experimental model of bacterial lung infection showed that Muc1−/− mice exhibited reduced lung colonization by P. aeruginosa, greater recruitment of leukocytes and higher levels of TNF-α and KC (mouse IL-8) in BALF compared with their wild type littermates. In vitro and in vivo mechanistic studies have indicated that MUC1/Muc1 plays an anti-inflammatory role during P. aeruginosa airway infection by suppressing TLR5 signaling [107–110]. More interestingly, the anti-inflammatory effect of MUC1/Muc1 was not limited to TLR5, but also included TLR2, 3, 4, 7 and 9, suggesting that this cell surface mucin may be a universal, negative regulator of TLR signaling [110]. Given that the host responses to lung pathogens involves the expression of multiple PAMPs, which must be activated and regulated in response to infection, this finding suggests a crucial role for MUC1/Muc1 in the resolution of inflammation, and perhaps in the genesis of chronic inflammatory disorders, such as COPD, CF and asthma.
Regulation of MUC1 Expression by TNF-α
Given the anti-inflammatory role of MUC1 in the airways, it is crucial to understand the mechanisms by which MUC1 gene expression is regulated. Several proinflammatory cytokines have been shown to up-regulate MUC1 expression. Noteworthy in this regard is TNF-α. Skerrett et al. [111] reported that TNFR1−/− mice treated intranasally with P. aeruginosa showed significantly increased airway inflammation compared with wild type mice, as measured by enhanced bacterial clearance from the lungs, increased numbers of neutrophils in BALF, and higher levels of TNF-α in BALF. Subsequently, TNF-α was demonstrated to stimulate MUC1 expression in A549 lung epithelial cells [107, 112]. The molecular mechanism of TNF-α-induced MUC1 up-regulation has been described in detail using a combination of biochemical, pharmacological, and molecular biological approaches [107]. The requirement for TNF-α in increased MUC1 expression has also been observed in A549 cells infected with respiratory syncytial virus (RSV) [109], as well as in mice infected with P. aeruginosa [113]. Thus, these results suggest that TNF-α may play a key role in controlling inflammation during airway infection, from the initiation phase of bacterial exposure to the final resolution of inflammation, the latter likely by inducing the expression of key anti-inflammatory molecules, such as MUC1, with possible assistance by IL-10 and/or PPAR-γ.
A Proposed Model for the Anti-Inflammatory Role of MUC1 in the Airways
Based on the accumulated published literature, we propose the following model to account for MUC1, TLRs, and TNF-α in the airway epithelial response to respiratory infection [25]. Normally, transiently inspired pathogens are quickly removed by mucociliary clearance and phagocytosis by resident leukocytes in the airway lumen. With abnormally high pathogen load, for example due to a predisposing condition such as CF, microbial PAMPs activate TLRs resulting in the production of pro-inflammatory mediators (IL-8 and TNF-α), thereby promoting leukocyte influx into the airways. During the early stage of infection, MUC1 expression is sufficiently low and TLR signaling is not antagonized. However, after invading pathogens have been cleared, increased levels of inflammatory products such as neutrophil elastase and TNF-α up-regulate MUC1 expression which, in turn, suppresses the release of TNF-α, thus inhibiting TLR-dependent airway inflammation. The net effect facilitates pathogen removal and returns the lungs to homeostasis. Future experiments are needed to provide additional support for this proposed negative feed-back loop model system.
MUC1 Mucin and Chronic Inflammatory Lung Disease
TNF is the major pro-inflammatory molecule during airway infection. Ulich et al. [114] demonstrated that intratracheal LPS-induced neutrophilic inflammation in rats can be inhibited by intratracheal administration of soluble TNFR, suggesting that TNF/TNFR interaction plays a key role in LPS-induced airway neutrophilic inflammation. Interestingly, TNFR1 deficient mice not only failed to control either LPS or Pseudomonas aeruginosa-induced neutrophilic inflammation [111] but showed greater neutrophilic inflammation to the contrary. Recently Choi et al. [113] showed that Muc1−/− mice behaves exactly the similar way as TNFR deficient mice in response to airway Pseudomonas aeruginosa infection, i.e., an increased neutrophilc inflammation, compared with their WT Muc1+/+ mice. The relationship between TNFR and Muc1 can be explained by Koga et al. [107] who demonstrated that the levels of MUC1 are controlled by the TNF/TNFR signaling pathway. Thus, MUC1/Muc1 seems to be controlled mainly by TNF both in vivo [113] and in vitro [107]. This timely regulation of inflammation and its resolution has also been demonstrated in vivo between TNF and IL-10, in which the former induces the latter, one of the major anti-inflammatory molecule [115]. Thus, failure to induce sufficient levels of anti-inflammatory molecules in a timely manner during the course of lung inflammation will result in lung tissue damage, and the subsequent repair processes will result in lung remodeling, a major characteristic of inflammatory lung diseases such as COPD and CF. Whether other anti-inflammatory molecules are also controlled through the similar mechanism remains to be elucidated.
One of the interesting questions that arise from this study is why there are multiple anti-inflammatory molecules and how they interact with each other during airway infection. For example, it has been shown that MUC1 induces IL-10, an anti-inflammatory cytokine, in dendritic cells [116] and that the levels of IL-10 in the BALF of Muc1 KO mice were significantly greater following Pseudomonas aeruginosa infection as compared with those of Muc1+/+ mice (unpublished data), suggesting the possible collaboration between the two during inflammation. The same question may be applied to other anti-inflammatory molecules in the lung that have been reported recently, including CD44 [117], aryl hydrocarbon receptor [118] and various lipid mediators [119, 120]. Further studies are required to understand the functional relationships between the known anti-inflammatory molecules during the resolution of airway inflammation.
Summary and Conclusions
In summary, airway epithelial cells play a critical role in the pathogenesis of chronic inflammatory lung disease. Their primary role in the process of host defense becomes dysregulated, and the excess inflammation causes increased mucus production and hypersecretion, resulting in mucostasis, airway obstruction, and tissue remodeling from several downstream events. Clinical consequences include an accelerated decline in lung function, greater respiratory symtoms, exacerbations of underlying lung disease, recurrent lower respiratory tract infection, and higher mortality. Multiple complex interactions between inflammatory cytokines and epithelial cells exist, and the precise roles of each in the generation of mucins and the amplification of lung inflammation remain unclear. There is, however, emerging evidence that the role of MUC1 mucin is essential to the airway epithelium’s response to environmental toxic agents, and therefore essential to the development of chronic and persistent inflammation. Further studies are required to better understand the roles of this mucin as well as others in the pathogenesis of inflammatory lung disease.
References
Lillehoj EP, Kim KC (2002) Airway mucus: its components and function. Arch Pharm Res 25:770–780
Voynow JA, Rubin BK (2009) Mucins, mucus, and sputum. Chest 135(2):505–512
Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G et al (2009) Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296(1):L92–L100
Ali M, Lillehoj EP, Park Y, Kyo Y, Kim KC (2011) Analysis of the proteome of human airway epithelial secretions. Proteome Sci 9:4
Zhang P, Summer WR, Bagby GJ, Nelson S (2000) Innate immunity and pulmonary host defense. Immunol Rev 173:39–51
Fahy JV, Dickey BF (2010) Airway mucus function and dysfunction. N Engl J Med 363(23):2233–2247
Salathe M (2007) Regulation of mammalian ciliary beating. Annu Rev Physiol 69:401–422
Knowles MR, Boucher RC (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109(5):571–577
Melloni B, Germouty J (1992) The influence of a new beta agonist: formoterol on mucociliary function. Rev Mal Respir 9(5):503–507
Gatto LA (1993) Cholinergic and adrenergic stimulation of mucociliary transport in the rat trachea. Respir Physiol 92(2):209–217
Lazarowski ER, Boucher RC (2009) Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9(3):262–267
Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC (2007) Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol 120(6):1279–1284
Henke MO, Shah SA, Rubin BK (2005) The role of airway secretions in COPD–clinical applications. COPD 2(3):377–390
Wanner A, Salathe M, O’Riordan TG (1996) Mucociliary clearance in the airways. Am J Respir Crit Care Med 154(6 Pt 1):1868–1902
Canning BJ (2006) Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest 129(1 Suppl):33S–47S
Rubin BK (2010) The role of mucus in cough research. Lung 188(Suppl 1):S69–S72
Sethi S, Murphy TF (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359(22):2355–2365
Schwab UE, Wold AE, Carson JL, Leigh MW, Cheng PW, Gilligan PH et al (1993) Increased adherence of Staphylococcus aureus from cystic fibrosis lungs to airway epithelial cells. Am Rev Respir Dis 148(2):365–369
Davis PB, Drumm M, Konstan MW (1996) Cystic fibrosis. Am J Respir Crit Care Med 154(5):1229–1256
Gilligan PH (1991) Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev 4(1):35–51
Prescott E, Lange P, Vestbo J (1995) Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 8(8):1333–1338
Kim V, Rogers TJ, Criner GJ (2008) New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 5(4):478–485
Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE et al (2000) Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 161(3 Pt 1):1016–1021
Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC et al (2006) Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 130(4):1102–1108
Kim KC, Lillehoj EP (2008) MUC1 mucin: a peacemaker in the lung. Am J Respir Cell Mol Biol 39(6):644–647
Kim V, Kelemen SE, Abuel-Haija M, Gaughan J, Sharafkhaneh A, Evans CM et al (2008) Small airway mucous metaplasia and inflammation in chronic obstructive pulmonary disease. JCOPD 5(6):329–338
Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC et al (2001) Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 164(3):469–473
Niewoehner DE, Kleinerman J, Rice DB (1974) Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 291(15):755–758
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L et al (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350(26): 2645–2653
Burgel PR, Nadel JA (2004) Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 59(11):992–996
James AL, Wenzel S (2007) Clinical relevance of airway remodelling in airway diseases. Eur Respir J 30(1):134–155
Macklem PT, Proctor DF, Hogg JC (1970) The stability of peripheral airways. Respir Physiol 8(2):191–203
Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC (2005) Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med 171(1):40–47
Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD et al (2007) Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 176(5):454–459
Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H (2006) Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 130(4):1129–1137
National Heart Lung and Blood Institute, National Institutes of Health (2001) Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guidelines, Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease: NHLMI/WHO Workshop Report. 2006. www.goldcopd.com, 2001
O’Donnell RA, Richter A, Ward J, Angco G, Mehta A, Rousseau K et al (2004) Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 59(12):1032–1040
Reid LM (1954) Pathology of chronic bronchitis. Lancet 266(6806):274–278
Kim WD, Ling SH, Coxson HO, English JC, Yee J, Levy RD et al (2007) The association between small airway obstruction and emphysema phenotypes in COPD. Chest 131(5): 1372–1378
Sciurba F, Martinez FJ, Rogers RM, Make B, Criner GJ, Cherniak RM et al (2006) The effect of small airway pathology on survival following lung volume reduction surgery (LVRS). [abstract]. Proc Am Thorac Soc 3:A712
Sherman CB, Xu X, Speizer FE, Ferris BG Jr, Weiss ST, Dockery DW (1992) Longitudinal lung function decline in subjects with respiratory symptoms. Am Rev Respir Dis 146(4):855–859
Vestbo J, Prescott E, Lange P (1996) Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153(5):1530–1535
Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE et al (2011) The chronic bronchitic phenotype of COPD: an analysis of the COPDGene study. Chest 140(3):626–633
Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carre P, Perez T et al (2009) Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 135(4):975–982
Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R et al (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363(12):1128–1138
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA (1998) Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(5 Pt 1):1418–1422
Niewoehner DE, Lokhnygina Y, Rice K, Kuschner WG, Sharafkhaneh A, Sarosi GA et al (2007) Risk indexes for exacerbations and hospitalizations due to COPD. Chest 131(1): 20–28
Kim V, Garfield JL, Grabianowski CL, Krahnke JS, Gaughan JP, Jacobs MR et al (2011) The effect of chronic sputum production on respiratory symptoms in severe COPD. COPD 8(2):114–120
Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD (2009) Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 64(10):894–900
Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ (2008) Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 5(4):549–555
James AL, Pare PD, Hogg JC (1989) The mechanics of airway narrowing in asthma. Am Rev Respir Dis 139(1):242–246
Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM (1991) Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis 143(1):138–143
Carroll N, Elliot J, Morton A, James A (1993) The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 147(2):405–410
Bergeron C, Boulet LP (2006) Structural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulation. Chest 129(4):1068–1087
Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM (2006) Airway mucus: from production to secretion. Am J Respir Cell Mol Biol 34(5):527–536
Dunnill MS (1960) The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 13:27–33
Huber HL, Koessler KK (1922) The pathology of bronchial asthma. Arch Intern Med 30: 689–760
Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM et al (2001) Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 163(2):517–523
Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS (1976) The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol 7(2):195–204
Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86(1):245–278
Evans CM, Kim K, Tuvim MJ, Dickey BF (2009) Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15(1):4–11
Caramori G, Casolari P, Di Gregorio C, Saetta M, Baraldo S, Boschetto P et al (2009) MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology 55(3):321–331
Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK (2004) MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am J Respir Cell Mol Biol 31(1):86–91
Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I (1999) Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 344(Pt 2):321–330
Takeda K, Akira S (2001) Roles of Toll-like receptors in innate immune responses. Genes Cells 6(9):733–742
Vogel SN, Fitzgerald KA, Fenton MJ (2003) TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv 3(8):466–477
Adamo R, Sokol S, Soong G, Gomez MI, Prince A (2004) Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 30(5):627–634
Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A et al (2004) Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30(6):777–783
Hajjar AM, O’Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ et al (2001) Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol 166(1):15–19
Mizel SB, Honko AN, Moors MA, Smith PS, West AP (2003) Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol 170(12):6217–6223
Soong G, Reddy B, Sokol S, Adamo R, Prince A (2004) TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest 113(10):1482–1489
Schroeder TH, Zaidi T, Pier GB (2001) Lack of adherence of clinical isolates of Pseudomonas aeruginosa to asialo-GM(1) on epithelial cells. Infect Immun 69(2):719–729
Burg ND, Pillinger MH (2001) The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol 99(1):7–17
Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN et al (1998) Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 4(5):615–618
Shapiro SD (2002) Neutrophil elastase: path clearer, pathogen killer, or just pathologic? Am J Respir Cell Mol Biol 26(3):266–268
Takayama M, Ishibashi M, Ishii H, Kuraki T, Nishida T, Yoshida M (2001) Effects of neutrophil elastase inhibitor (ONO-5046) on lung injury after intestinal ischemia-reperfusion. J Appl Physiol 91(4):1800–1807
Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM (1999) Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol 276(5 Pt 1):L835–L843
Kim KC, Lee BC, Pou S, Ciccolella D (2003) Effects of activation of polymorphonuclear leukocytes on airway goblet cell mucin release in a co-culture system. Inflamm Res 52(6): 258–262
Kohri K, Ueki IF, Nadel JA (2002) Neutrophil elastase induces mucin production by ligand-dependent epidermal growth factor receptor activation. Am J Physiol Lung Cell Mol Physiol 283(3):L531–L540
Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB (2005) Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol 167(3):651–661
Denning GM, Stoll LL (2006) Peroxisome proliferator-activated receptors: potential therapeutic targets in lung disease? Pediatr Pulmonol 41(1):23–34
Nichols D, Chmiel J, Berger M (2008) Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 34(2):146–162
Sabat R (2010) IL-10 family of cytokines. Cytokine Growth Factor Rev 21(5):315–324
Barcelo B, Pons J, Fuster A, Sauleda J, Noguera A, Ferrer JM et al (2006) Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 145(3):474–479
Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P (2010) Regulatory T cells in many flavors control asthma. Mucosal Immunol 3(3):216–229
Lloyd CM, Hawrylowicz CM (2009) Regulatory T cells in asthma. Immunity 31(3): 438–449
Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T et al (2007) Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol 178(3):1433–1442
Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C et al (2000) Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med 192(2):151–158
Konigshoff M, Kneidinger N, Eickelberg O (2009) TGF-beta signaling in COPD: deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly 139(39–40):554–563
Lee JY, Elmer HL, Ross KR, Kelley TJ (2004) Isoprenoid-mediated control of SMAD3 expression in a cultured model of cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 31(2):234–240
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R et al (1997) The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 272(30):18779–18789
Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B et al (1996) Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97(11):2553–2561
Giaginis C, Giagini A, Theocharis S (2009) Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands as potential therapeutic agents to treat arthritis. Pharmacol Res 60(3):160–169
Belvisi MG, Hele DJ, Birrell MA (2006) Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol 533(1–3):101–109
Ward JE, Tan X (2007) Peroxisome proliferator activated receptor ligands as regulators of airway inflammation and remodelling in chronic lung disease. PPAR Res 2007:14983
Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB (2008) Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol 295(2):L303–L313
Vanden Berghe W, Vermeulen L, Delerive P, De Bosscher K, Staels B, Haegeman G (2003) A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol 544:181–196
Ollero M, Junaidi O, Zaman MM, Tzameli I, Ferrando AA, Andersson C et al (2004) Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol 200(2):235–244
Reynders V, Loitsch S, Steinhauer C, Wagner T, Steinhilber D, Bargon J (2006) Peroxisome proliferator-activated receptor alpha (PPAR alpha) down-regulation in cystic fibrosis lymphocytes. Respir Res 7:104
Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J et al (1990) Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem 265(25):15286–15293
Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA (1990) Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem 265(25):15294–15299
Parry S, Silverman HS, McDermott K, Willis A, Hollingsworth MA, Harris A (2001) Identification of MUC1 proteolytic cleavage sites in vivo. Biochem Biophys Res Commun 283(3):715–720
Zrihan-Licht S, Baruch A, Elroy-Stein O, Keydar I, Wreschner DH (1994) Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins. Cytokine receptor-like molecules. FEBS Lett 356(1):130–136
Spicer AP, Rowse GJ, Lidner TK, Gendler SJ (1995) Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem 270(50):30093–30101
Kardon R, Price RE, Julian J, Lagow E, Tseng SC, Gendler SJ et al (1999) Bacterial conjunctivitis in Muc1 null mice. Invest Ophthalmol Vis Sci 40(7):1328–1335
Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP et al (2006) Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 176(7):3890–3894
Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y et al (2007) TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 293(3):L693–L701
Kato K, Lu W, Kai H, Kim KC (2007) Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol 293(3):L686–L692
Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC (2010) Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 298(4):L558–L563
Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H et al (2008) MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 38(3):263–268
Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB (1999) Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol 276(5 Pt 1):L715–L727
Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC (2007) The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol 37(6):691–698
Choi S, Park YS, Koga T, Treloar A, Kim KC (2011) TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol 44(2):255–260
Ulich TR, Yin S, Remick DG, Russell D, Eisenberg SP, Kohno T (1993) Intratracheal administration of endotoxin and cytokines. IV. The soluble tumor necrosis factor receptor type I inhibits acute inflammation. Am J Pathol 142(5):1335–1338
Cox G (1996) IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol 271(4 Pt 1):L566–L571
Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V et al (2004) Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol 172(12):7341–7349
Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X et al (2007) CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol 178(4):2469–2475
Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP et al (2007) Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol 170(3):855–864
Zhou W, Hashimoto K, Goleniewska K, O’Neal JF, Ji S, Blackwell TS et al (2007) Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol 178(2):702–710
Bonnans C, Levy BD (2007) Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol 36(2):201–205
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kim, V., Kato, K., Kim, K.C., Lillehoj, E.P. (2013). Role of Epithelial Cells in Chronic Inflammatory Lung Disease. In: Rogers, T., Criner, G., Cornwell, W. (eds) Smoking and Lung Inflammation. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7351-0_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7351-0_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7350-3
Online ISBN: 978-1-4614-7351-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)