Abstract
In growing eukaryotic cells, nuclear ribosomal (r)RNA synthesis by RNA polymerase (RNAP) I accounts for the vast majority of cellular transcription. This high output is achieved by the presence of multiple copies of rRNA genes in eukaryotic genomes transcribed at a high rate. In contrast to most of the other transcribed genomic loci, actively transcribed rRNA genes are largely devoid of nucleosomes adapting a characteristic “open” chromatin state, whereas a significant fraction of rRNA genes resides in a transcriptionally inactive nucleosomal “closed” chromatin state. Here, we review our current knowledge about the nature of open rRNA gene chromatin and discuss how this state may be established.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- Nucleolus
- Nucleolar organizer region (NOR)
- Ribosomal DNA
- Ribosomal RNA genes
- RNA polymerase I
- Transcription
- Chromatin
- Nucleosome
- Preinitiation complex (PIC)
- High mobility group (HMG) box proteins
- Upstream binding factor (UBF)
- Hmo1
- Psoralen cross-linking
- Chromatin immunoprecipitation (ChIP )
- Chromatin endogenous cleavage (ChEC)
- Electron microscopy (EM)
1 Introduction
This short review aims at summarizing research which has contributed to our current understanding of characteristic chromatin states at eukaryotic rRNA gene loci sharing many conserved features from yeast to human. This subject has also (partly) be covered by other reviews in the past, which are recommended for further reading [1,2,3,4,5]. The regulation of RNAP I transcription by epigenetic mechanisms (including posttranslational covalent modifications of histones and other components of the RNAP I transcription machinery, as well as DNA-methylation ) has been reviewed in great detail in the past [6,7,8,9] and will not be subject of this article.
In the nucleus of eukaryotic cells, the genetic information encoded in the DNA is assembled in the structure of chromatin . The basic unit of chromatin is called the nucleosome and consists of approximately 146 bp of DNA wrapped around a histone octamer (reviewed in [10,11,12]). The tight wrapping of the nucleic acid around the protein core renders the DNA in part inaccessible which plays an important role for the regulation of essential nuclear processes like replication, DNA-repair, and transcription . To deal with nucleosomal DNA, eukaryotic cells developed mechanisms altering chromatin structure at distinct loci in specific situations (reviewed in [13, 14]). Accordingly, characteristic changes in chromatin structure and posttranslational covalent modification state of chromatin components correlate with transitions in the transcriptional status of individual genes. Understanding the relationship between chromatin structure and transcription will be essential to fully comprehend the complex process of eukaryotic gene expression .
2 Visualization of rRNA Transcription
2.1 Actively Transcribed rRNA Genes Are Prominent Structures in Nuclear Chromatin Spreads
In eukaryotes , there are at least three different nuclear RNAPs, numbered I–III, each of which has a distinct set of target genes (reviewed in [15], see also short reviews by Merkl et al. and Pilsl et al., this issue). Whereas RNAP II transcribes all protein coding genes, RNAP III is dedicated to the synthesis of small noncoding RNAs including the 5S rRNA and tRNAs . In all organisms—with only one known exception [16]—RNAP I has only one acknowledged genomic target locus from which it synthesizes a large precursor transcript encompassing the sequences of three out of four rRNAs (hereafter called rRNA gene). Remarkably, rRNAs produced by RNAP I account for more than 60% of cellular RNA synthesis in exponentially growing S. cerevisiae cells (hereafter called yeast , reviewed in [17]). This is achieved by a strong promoter with a stably bound preinitiation complex (PIC) (reviewed in [18]), as well as the multimerization of the genomic templates (reviewed in [19]). Thus, rRNA genes exist as clusters of repeated transcription units, the so-called nucleolar organizer regions (NORs), at one or more genomic locations depending on the organism. Actively transcribed NORs are part of the nucleolus , the dominant nuclear substructure of early ribosome biogenesis , whereas inactive NORs do not associate with nucleoli (reviewed in [20]).
Even slightly before the different nuclear RNAPs were biochemically defined [21, 22], it was possible to visualize the enzymes transcribing their target loci in chromatin spreads by electron microscopy (EM) [23,24,25]. The first transcription units that were unambiguously identified by this method were the actively transcribed extrachromosomal ribosomal RNA genes isolated from amphibian oocytes [25]. These transcription units showed a characteristic “Christmas-tree ” like appearance with tightly packed elongating RNAPs forming the stem, and protein-coated nascent rRNAs extending from the polymerases forming the branches of the trees. Depending on the preparation, Christmas trees can be decorated with “terminal balls” representing preribosomal assembly intermediates [26]. Ever since, rRNA gene Christmas-trees have been observed in chromatin spreads from different cell types of many organisms (reviewed in [27, 28]). These studies yielded important insights in aspects of RNAP I transcription at the single molecule level. More recently, the spreading technique combined with cryo-EM tomography allowed to obtain more detailed structural information about yeast RNAP I transcribing its native template [29]. In fact, shortly after the first Christmas trees had been described, the conserved repeated “beads-on-the-string” nature of nontranscribed eukaryotic chromatin came into the focus of the researchers [30, 31]. The beads-on-the-string seen in EM were biochemically defined as complexes of DNA and histone octamers, the fundamental units of chromatin named nucleosomes [32,33,34].
2.2 rRNA Genes Transcribed by RNAP I Are Nucleosome Depleted
The above analyses of chromatin spreads provided first insights into how different RNAPs deal with the chromatin template (reviewed in [27]). Thus, nonribosomal chromatin moderately transcribed by (presumably) RNAP II remained at least partially covered with nucleosomes [35,36,37]. This indicated that nucleosomes may either persist or are reestablished upon RNAP II transcription . In contrast, there was evidence that RNAP I and RNAP III transcription occurred exclusively on nucleosome depleted templates even in situations when transcription rate was reduced (reviewed in [27, 38,39,40]). Complementing EM analyses, endonucleases were used as molecular probes to investigate chromatin structure (reviewed in [41,42,43]). Endonucleases can only poorly access DNA assembled into a nucleosome . Therefore, endonuclease cleavage at a genomic region of interest can be used to deduce information about local nucleosome occupancy. In such assays, DNA in actively transcribed rRNA gene chromatin was more accessible than DNA in nontranscribed rRNA gene chromatin , supporting the view of nucleosome depletion at RNAP I transcribed regions [44, 45]. This notion was corroborated by in vivo and in vitro cross-linking of chromosomal DNA with psoralen (reviewed in [46] and references therein). Psoralen is a parent compound of naturally occurring substances which can intercalate in DNA (reviewed in [47] and references therein). Upon exposure to longwave ultraviolet (UVA) radiation, psoralen incorporation leads to DNA-interstrand cross-links. As observed for nucleases, nucleosome formation prevents psoralen intercalation into nucleosomal DNA tightly interacting with the histone octamer [48, 49]. Therefore, psoralen cross-links are restricted to accessible nucleosome-free DNA regions. After DNA isolation from psoralen cross-linked chromatin , nucleosomes leave a characteristic footprint of approximately 146 bp of non–cross-linked DNA surrounded by cross-linked linker DNA. This can be analyzed by EM of the cross-linked DNA under denaturing conditions, in which the non–cross-linked DNA regions are visualized as single stranded DNA bubbles . Consistent with nucleosome depletion, DNA isolated from psoralen treated actively transcribed rRNA gene chromatin was heavily cross-linked and largely devoid of single-stranded DNA bubbles [50].
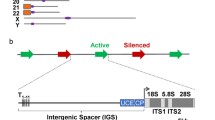
2.3 rRNA Genes Coexist in At Least Two Different Chromatin States
Psoralen cross-linking alters the mobility of deproteinized DNA fragments in native agarose gel electrophoresis [50]. A high-degree of psoralen incorporation in nucleosome-depleted DNA leads to a strong retardation of the corresponding fragment, whereas lower psoralen incorporation in nucleosomal DNA yields faster migrating fragments. In combination with Southern blot analysis this technique can be used to monitor nucleosome occupancy at specific restriction fragments obtained from psoralen cross-linked chromosomal DNA [50,51,52]. DNA-fragments deriving from psoralen cross-linked transcribed regions of rRNA genes from a human cell line and yeast cells migrated as two major bands with low and high mobility in native agarose gel electrophoresis, indicating that these genes adapt at least two different chromatin states [51, 52]. Nascent RNA was exclusively cross-linked to the fragment of low mobility, suggesting that nucleosome occupancy at actively transcribed rRNA genes is strongly decreased [51, 52]. This led to the model that rRNA genes may coexist at least in a nucleosome depleted, “open” chromatin state and a transcriptionally inactive nucleosomal “closed” chromatin state. It should be noted, that psoralen cross-linking measures nucleosome occupancy at selected loci, but does not allow straightforward conclusions about the transcriptional state of a gene (reviewed in [2, 3] and references therein). Therefore, open rRNA genes are not necessarily actively transcribed, although actively transcribed rRNA genes appear to be always in an open chromatin state . To date, no other chromosomal locus with a psoralen accessibility similar to open rRNA genes has been identified. Strikingly, psoralen cross-linking of heavily transcribed RNAP II-dependent genes did not yield fragments with the low mobility expected for fully cross-linked DNA [53]. This may corroborate the observations in EM that RNAP II transcribed gene regions retain a significant number of nucleosomal particles [35, 37]. Thus, the open rRNA gene chromatin state is probably unique regarding the extent of nucleosome depletion and the size of the nucleosome depleted region.
With the advent of chromatin immunoprecipitation (ChIP) the nucleosome-depleted nature of actively transcribed rRNA genes was challenged (reviewed in [2, 3]). In ChIP experiments, histone molecules coprecipitated rRNA gene fragments from extracts obtained from yeast cells carrying a reduced number of rRNA gene copies [54]. Because under these conditions, the majority of rRNA genes were transcribed [55], it was concluded that RNAP I transcribes a dynamic, nucleosomal chromatin template. A subsequent study based on chromatin endogenous cleavage (ChEC) experiments in yeast supported rather the model of robust histone /nucleosome depletion at actively transcribed rRNA genes [56]. ChEC is performed in yeast strains in which a protein of interest is expressed in fusion with Micrococcal nuclease (MNase) from Staphylococcus aureus [57]. MNase is a secreted DNA and RNA endo- and exonuclease which has long been used to study chromatin structure (reviewed in [41, 43], see chapter of Teubl et al. in this issue). MNase activity strictly depends on calcium. Thus, due to the low intracellular calcium concentrations recombinantly expressed MNase fusion proteins are not active. After isolation of crude nuclei from cells expressing MNase-fusion proteins and addition of calcium, the tethered nuclease will cut neighboring DNA. Specific cleavage events in genomic DNA can be monitored by Southern blot analysis, primer extension or high-throughput sequencing to reveal which DNA regions were in the proximity of the MNase fusion proteins [58, 59]. ChEC experiments can also be performed in combination with psoralen cross-linking to determine if a factor of interest preferentially associates with the open or the closed rRNA gene chromatin state [56, 60]. Using this method, it could be shown that RNAP I subunits fused to MNase specifically degraded the highly psoralen cross-linked fragments derived from open rRNA gene chromatin in yeast . In contrast, histone-MNase fusion proteins preferentially degraded the poorly psoralen cross-linked fragments derived from closed rRNA gene chromatin . These results supported the hypothesis that RNAP I transcribes a histone /nucleosome depleted chromatin template [56]. The apparent contradiction of these results to the conclusions derived from ChIP experiments [54] may be explained by the notion that the term histone depletion does not exclude that a few histone molecules occasionally associate with open rRNA gene chromatin . Additionally, even in yeast cells with a lower rRNA gene copy number, a small subpopulation of rRNA genes resides in the closed nontranscribed nucleosomal chromatin state [55, 61]. This nucleosomal subpopulation might then be detected in ChIP analyses, which—unlike the combination of ChEC with psoralen cross-linking analyses—does not distinguish between DNA fragments derived from open or closed rRNA gene chromatin . In higher eukaryotes ChEC combined with psoralen cross-linking analyses have not been conducted so far. Nevertheless, endonuclease accessibility of human rRNA gene loci combined with high throughput sequencing (HTS) suggested that nucleosome occupancy is reduced at rRNA genes when compared to intergenic regions [62]. More recently, a deconvolution histone ChIP-HTS analysis combined with genetic manipulation of RNAP I transcription in mice strongly supported that histone depletion at active rRNA genes is conserved from yeast to mammals [63, 64].
2.4 HMG-Box Proteins Are Architectural Components of Open rRNA Gene Chromatin States
The high mobility group (HMG) box is a structural motif first described in eukaryotic proteins with a high electrophoretic mobility (reviewed in [65]). HMG box proteins are involved in the regulation of many important DNA-dependent processes in the nucleus presumably due to their acknowledged function as architectural chromatin components. Along these lines, HMG-box proteins are conserved constituents of open rRNA gene chromatin (reviewed in [2, 63, 66, 67]). In vertebrates, the upstream binding factor (UBF) was initially identified to be required for proper recruitment of the basal RNAP I PIC at the rRNA gene promoter [68,69,70]. UBF has a characteristic arrangement of up to six tandemly repeated HMG-boxes (reviewed in [71]). Upon dimerization UBF may wrap DNA in one single loop in a structure called the “enhancesome ” in vitro [72]. It was suggested that this structure establishes a characteristic architecture at the rRNA gene promoter (reviewed in [71]). In vivo, UBF molecules spread along the entire rRNA gene region transcribed by RNAP I [62, 63, 66, 73, 74]. Additionally, UBF is preferentially recruited to repetitive enhancer DNA elements preceding the rRNA gene promoter in higher eukaryotes [62, 63, 69, 73, 74]. UBF was shown to localize to NORs harboring active rRNA genes [75]. During the cell division cycle, UBF stays associated with NORs which have been transcriptionally active even upon RNAP I transcription shutdown at mitosis [76,77,78]. In this condition, UBF likely maintains NORs bearing active rRNA gene clusters in a hypocondensed, open chromatin state leading to “secondary constrictions” visualized by light microscopy in plant mitotic chromosomes early in the twentieth century [79,80,81]. Yeast contains a bona fide UBF homologue Hmo1 [82, 83]. As UBF , Hmo1 is a chromatin component of actively transcribed rRNA gene regions but has only one HMG-box and—in contrast to UBF —no reported role in RNAP I PIC formation [56, 84,85,86]. Both murine UBF and yeast Hmo1 are required to maintain the nucleosome depleted open rRNA gene chromatin state in the absence of RNAP I transcription [61, 63]. In agreement, both UBF and Hmo1 can destabilize nucleosomal templates in vitro [87, 88]. Furthermore, Hmo1 mediated DNA compaction, bridging and looping observed in vitro was suggested to be implicated in the maintenance of the nucleosome depleted rRNA gene chromatin state in vivo [89]. UBF and Hmo1 bind to various DNA sequences in vitro [90,91,92] and genome wide ChIP analyses suggested that both proteins additionally associate with nucleosome depleted promoter regions of highly transcribed RNAP II dependent genes [84,85,86, 93]. This indicates that UBF and Hmo1 may generally recognize structures associated with highly transcribed DNA templates. This hypothesis was substantiated in in vitro studies in which Hmo1 bound preferentially to DNA templates thought to mimic transcription intermediates [91]. Along these lines it has also been suggested that Hmo1 protects negatively supercoiled DNA at gene boundaries in vivo [94], indicating that (r)DNA topology may be important for Hmo1 recruitment. Whereas there is evidence that recruitment of Hmo1 to rRNA gene sequences is dependent on prior RNAP I transcription [61, 86], RNAP I transcription may not be required to recruit UBF to the rRNA gene promoter or enhancer elements [95]. Thus, integration of rRNA gene enhancer repeats from Xenopus laevis at different loci in human chromosomes led to the formation of “pseudo-NORs” resulting in secondary constrictions in mitotic chromosomes (reviewed in [67, 96]). These structures were bound by human UBF , other components of the basal RNAP I transcription machinery and ribosome biogenesis factors [95, 97] but appear to be transcriptional inactive. Although, psoralen accessibility at pseudo-NOR sequences has not been tested to date, it is assumed that they reside in an open chromatin structure . Taken together, both Hmo1 and UBF are architectural proteins of open rRNA gene chromatin and involved in the maintenance of the nucleosome depleted state [56, 61, 63, 73]. Additionally, UBF binding at the rRNA gene promoter (and likely at enhancer regions) is likely related to its role in RNAP I PIC formation (reviewed in [18, 66]). As an essential PIC component, UBF —but not Hmo1 —is required for the establishment of the open rRNA gene chromatin state in vivo [56, 63, 73].
2.5 Molecular Requirements to Establish the Open rRNA Gene Chromatin State
As indicated above, there is a tight correlation between RNAP I transcription and the establishment of the open chromatin state . In fact, studies in yeast indicated that the equilibrium of open and closed chromatin states observed in asynchronously dividing cells may be explained as the result of RNAP I transcription dependent opening and replication dependent closing of rRNA genes [61]. In each synthesis phase of the cell division cycle, replication leads to nucleosome deposition and chromatin closing at rRNA genes on both sister chromatids [98]. After rRNA genes have been replicated, opening of rRNA genes correlates with the onset of RNAP I transcription . In higher eukaryotes , cell division cycle dependent rRNA gene chromatin transitions were likely dismissed in early psoralen cross-linking studies in which interphase cells were compared with metaphase cells [51]. However at least in one study, time course experiments during the cell division cycle in a human cell line revealed very similar rRNA gene chromatin state transitions to those observed in yeast [99]. In agreement with a requirement for RNAP I transcription for establishment of the open rRNA gene chromatin state in replicating yeast cells, all rRNA genes adapt the closed chromatin state when RNAP I transcription is impaired [61]. In addition, there was good correlation between downregulation of RNAP I transcription and closing of rRNA gene chromatin in various physiological relevant situations. Thus, rRNA gene chromatin closing occurs when cells grow to stationary phase [51, 52, 100, 101], upon UV-damage [102, 103], or when cells differentiate [104]. On the other hand, upregulation of RNAP I transcription upon transfer of stationary yeast cells in fresh growth media correlates with rapid opening of rRNA genes [100, 101]. Furthermore, the observed opening of rRNA genes when replication-mediated chromatin closing was prevented strictly depends on ongoing RNAP I transcription [61].
It remains unclear if RNAP I transcription alone suffices to establish open, nucleosome-depleted rRNA gene chromatin . Thus, additional factors may assist RNAP I to convert genes from the closed to the open chromatin state . The “Facilitates Chromatin Transcription ” (FACT ) complex, known to support RNAP II transcription , was shown to associate with rRNA genes in yeast and human, copurified with human RNAP I and enhanced RNAP I nonspecific transcription from chromatin templates in vitro [39, 105]. In yeast , the chromatin remodeling factors Ino80, Isw1, and Isw2, as well as the Swi/Snf complex associate with rRNA genes in vivo and ex vivo and may modulate rRNA gene chromatin structure [106,107,108]. Furthermore, bona fide RNAP II transcription (elongation ) factors TFIIH , Paf1 , Spt4/5 , Spt6 , and THO were reported to support RNAP I transcription [109,110,111,112,113,114]. In addition, early ribosome biogenesis factors might be components of rRNA gene chromatin in yeast and higher eukaryotes perhaps supporting efficient RNAP I transcription [97, 115]. Finally, a constantly growing number of factors which are involved in (epigenetic) posttranslational covalent modifications of chromatin components (including proteins and nucleic acids), as well as noncoding RNAs may influence RNAP I transcription and the establishment of rRNA gene chromatin states (reviewed in [6,7,8,9]). Since most of the above factors have acknowledged roles in various nuclear processes, it is, however, often difficult to distinguish between direct and indirect effects on RNAP I transcription and rRNA gene chromatin structure .
3 Conclusions
Our current knowledge of molecular mechanisms required for establishment of the open rRNA gene chromatin state is mostly based on in vivo observations or ex vivo analyses of relatively crude cellular fractions. In the future, analyses with defined in vitro reconstituted chromatin will probably allow to stringently test current hypotheses and to describe molecular mechanisms which are responsible for chromatin opening (see [116] and chapter by Merkl et al., this issue). One promising approach may involve the investigation of isolated native rRNA gene chromatin templates. Chromosomal rRNA gene domains in their native chromatin context can be purified from yeast , and initial analyses indicate that important features of the in vivo chromatin structure are maintained upon isolation [107]. The purified chromatin can be subjected to functional biochemical assays, or single molecule structural analyses [107, 117]. The reconstitution of transcription using highly purified components of the RNAP I transcription machinery and the native chromatin template in vitro might closely reflect the in vivo situation. Together, these studies may help to derive a detailed understanding about the interplay between chromatin structure and transcription on a molecular level. Given the tight connection between RNAP I transcription and early steps in ribosome biogenesis [115], it should be attempted to include selected ribosome biogenesis factors in the purified system. Perhaps, this may lead us soon to the first Christmas trees “grown” in vitro.
References
Birch JL, Zomerdijk JCBM (2008) Structure and function of ribosomal RNA gene chromatin. Biochem Soc Trans 36:619–624
Hamperl S, Wittner M, Babl V, Perez-Fernandez J, Tschochner H, Griesenbeck J (2013) Chromatin states at ribosomal DNA loci. Biochim Biophys Acta 1829:405–417
Moss T, Mars J-C, Tremblay MG, Sabourin-Felix M (2019) The chromatin landscape of the ribosomal RNA genes in mouse and human. Chromosome Res 27(1–2):31–40. https://doi.org/10.1007/s10577-018-09603-9
Németh A, Längst G (2008) Chromatin organization of active ribosomal RNA genes. Epigenetics 3:243–245
Schöfer C, Weipoltshammer K (2018) Nucleolus and chromatin. Histochem Cell Biol 150:209–225
Drygin D, Rice WG, Grummt I (2010) The RNA polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol 50:131–156
Grummt I, Längst G (2013) Epigenetic control of RNA polymerase I transcription in mammalian cells. Biochim Biophys Acta 1829:393–404
Srivastava R, Srivastava R, Ahn SH (2016) The epigenetic pathways to ribosomal DNA silencing. Microbiol Mol Biol Rev 80:545–563
Sharifi S, Bierhoff H (2018) Regulation of RNA polymerase I transcription in development, disease, and aging. Annu Rev Biochem 87:51–73
Andrews AJ, Luger K (2011) Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys 40:99–117
Kornberg RD, Lorch Y (1999) Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98:285–294
Olins DE, Olins AL (2003) Chromatin history: our view from the bridge. Nat Rev Mol Cell Biol 4:809–814
Lai WKM, Pugh BF (2017) Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat Rev Mol Cell Biol 18:548–562
Rando OJ, Winston F (2012) Chromatin and transcription in yeast. Genetics 190:351–387
Vannini A, Cramer P (2012) Conservation between the RNA polymerase I, II, and III transcription initiation machineries. Mol Cell 45:439–446
Günzl A, Bruderer T, Laufer G, Schimanski B, Tu L-C, Chung H-M, Lee P-T, Lee MG-S (2003) RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot Cell 2:542–551
Warner JR (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 24:437–440
Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V (2007) A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci 64:29–49
Long EO, Dawid IB (1980) Repeated genes in eukaryotes. Annu Rev Biochem 49:727–764
McStay B (2016) Nucleolar organizer regions: genomic “dark matter” requiring illumination. Genes Dev 30:1598–1610
Kedinger C, Gniazdowski M, Mandel JL, Gissinger F, Chambon P (1970) Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun 38:165–171
Roeder RG, Rutter WJ (1969) Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature 224:234–237
Miller OL, Beatty BR (1969) Extrachromosomal nucleolar genes in amphibian oocytes. Genetics 61(Suppl):133–143
Miller OL, Beatty BR (1969) Portrait of a gene. J Cell Physiol 74(Suppl 1):225+
Miller OL, Beatty BR (1969) Visualization of nucleolar genes. Science 164:955–957
Mougey EB, O’Reilly M, Osheim Y, Miller OL, Beyer A, Sollner-Webb B (1993) The terminal balls characteristic of eukaryotic rRNA transcription units in chromatin spreads are rRNA processing complexes. Genes Dev 7:1609–1619
Scheer U (1987) Contributions of electron microscopic spreading preparations (“Miller spreads”) to the analysis of chromosome structure. Results Probl Cell Differ 14:147–171
Trendelenburg MF, Zatsepina OV, Waschek T, Schlegel W, Tröster H, Rudolph D, Schmahl G, Spring H (1996) Multiparameter microscopic analysis of nucleolar structure and ribosomal gene transcription. Histochem Cell Biol 106:167–192
Neyer S, Kunz M, Geiss C, Hantsche M, Hodirnau V-V, Seybert A, Engel C, Scheffer MP, Cramer P, Frangakis AS (2016) Structure of RNA polymerase I transcribing ribosomal DNA genes. Nature 540(7634):607–610. https://doi.org/10.1038/nature20561
Olins AL, Olins DE (1974) Spheroid chromatin units (v bodies). Science 183:330–332
Woodcock CL, Safer JP, Stanchfield JE (1976) Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp Cell Res 97:101–110
Kornberg RD (1974) Chromatin structure: a repeating unit of histones and DNA. Science 184:868–871
Kornberg RD, Thomas JO (1974) Chromatin structure; oligomers of the histones. Science 184:865–868
Oudet P, Gross-Bellard M, Chambon P (1975) Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell 4:281–300
Foe VE, Wilkinson LE, Laird CD (1976) Comparative organization of active transcription units in Oncopeltus fasciatus. Cell 9:131–146
Laird CD, Chooi WY (1976) Morphology of transcription units in Drosophila melanogaster. Chromosoma 58:193–218
Laird CD, Wilkinson LE, Foe VE, Chooi WY (1976) Analysis of chromatin-associated fiber arrays. Chromosoma 58:169–190
French SL, Osheim YN, Schneider DA, Sikes ML, Fernandez CF, Copela LA, Misra VA, Nomura M, Wolin SL, Beyer AL (2008) Visual analysis of the yeast 5S rRNA gene transcriptome: regulation and role of La protein. Mol Cell Biol 28:4576–4587
Johnson JM, French SL, Osheim YN, Li M, Hall L, Beyer AL, Smith JS (2013) Rpd3- and spt16-mediated nucleosome assembly and transcriptional regulation on yeast ribosomal DNA genes. Mol Cell Biol 33:2748–2759
Scheer U (1978) Changes of nucleosome frequency in nucleolar and non-nucleolar chromatin as a function of transcription: an electron microscopic study. Cell 13:535–549
Reeves R (1984) Transcriptionally active chromatin. Biochim Biophys Acta 782:343–393
Tsompana M, Buck MJ (2014) Chromatin accessibility: a window into the genome. Epigenetics Chromatin 7:33
Voong LN, Xi L, Wang J-P, Wang X (2017) genome-wide mapping of the nucleosome landscape by micrococcal nuclease and chemical mapping. Trends Genet 33:495–507
Reeves R (1978) Nucleosome structure of Xenopus oocyte amplified ribosomal genes. Biochemistry 17:4908–4916
Reeves R (1978) Structure of Xenopus ribosomal gene chromatin during changes in genomic transcription rates. Cold Spring Harb Symp Quant Biol 42(Pt 2):709–722
Toussaint M, Levasseur G, Tremblay M, Paquette M, Conconi A (2005) Psoralen photocrosslinking, a tool to study the chromatin structure of RNA polymerase I--transcribed ribosomal genes. Biochem Cell Biol Biochim Biol Cell 83:449–459
Hearst JE (1981) Psoralen photochemistry and nucleic acid structure. J Invest Dermatol 77:39–44
Hanson CV, Shen CK, Hearst JE (1976) Cross-linking of DNA in situ as a probe for chromatin structure. Science 193:62–64
Wieshahn GP, Hyde JE, Hearst JE (1977) The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry 16:925–932
Sogo JM, Ness PJ, Widmer RM, Parish RW, Koller T (1984) Psoralen-crosslinking of DNA as a probe for the structure of active nucleolar chromatin. J Mol Biol 178:897–919
Conconi A, Widmer RM, Koller T, Sogo JM (1989) Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell 57:753–761
Dammann R, Lucchini R, Koller T, Sogo JM (1993) Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res 21:2331–2338
Cavalli G, Thoma F (1993) Chromatin transitions during activation and repression of galactose-regulated genes in yeast. EMBO J 12:4603–4613
Jones HS, Kawauchi J, Braglia P, Alen CM, Kent NA, Proudfoot NJ (2007) RNA polymerase I in yeast transcribes dynamic nucleosomal rDNA. Nat Struct Mol Biol 14:123–130
French SL, Osheim YN, Cioci F, Nomura M, Beyer AL (2003) In exponentially growing Saccharomyces cerevisiae cells, rRNA synthesis is determined by the summed RNA polymerase I loading rate rather than by the number of active genes. Mol Cell Biol 23:1558–1568
Merz K, Hondele M, Goetze H, Gmelch K, Stoeckl U, Griesenbeck J (2008) Actively transcribed rRNA genes in S. cerevisiae are organized in a specialized chromatin associated with the high-mobility group protein Hmo1 and are largely devoid of histone molecules. Genes Dev 22:1190–1204
Schmid M, Durussel T, Laemmli UK (2004) ChIC and ChEC; genomic mapping of chromatin proteins. Mol Cell 16:147–157
Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK (2006) Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol Cell 21:379–391
Zentner GE, Kasinathan S, Xin B, Rohs R, Henikoff S (2015) ChEC-seq kinetics discriminates transcription factor binding sites by DNA sequence and shape in vivo. Nat Commun 6:8733
Griesenbeck J, Wittner M, Charton R, Conconi A (2012) Chromatin endogenous cleavage and psoralen crosslinking assays to analyze rRNA gene chromatin in vivo. Methods Mol Biol Clifton NJ 809:291–301
Wittner M, Hamperl S, Stöckl U, Seufert W, Tschochner H, Milkereit P, Griesenbeck J (2011) Establishment and maintenance of alternative chromatin states at a multicopy gene locus. Cell 145:543–554
Zentner GE, Saiakhova A, Manaenkov P, Adams MD, Scacheri PC (2011) Integrative genomic analysis of human ribosomal DNA. Nucleic Acids Res 39:4949–4960
Herdman C, Mars J-C, Stefanovsky VY, Tremblay MG, Sabourin-Felix M, Lindsay H, Robinson MD, Moss T (2017) A unique enhancer boundary complex on the mouse ribosomal RNA genes persists after loss of Rrn3 or UBF and the inactivation of RNA polymerase I transcription. PLoS Genet 13:e1006899
Mars J-C, Sabourin-Felix M, Tremblay MG, Moss T (2018) A deconvolution protocol for ChIP-Seq reveals analogous enhancer structures on the mouse and human ribosomal RNA genes. G3 (Bethesda) 8:303–314
Stros M, Launholt D, Grasser KD (2007) The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell Mol Life Sci 64:2590–2606
Sanij E, Hannan RD (2009) The role of UBF in regulating the structure and dynamics of transcriptionally active rDNA chromatin. Epigenetics 4:374–382
Wright JE, Mais C, Prieto J-L, McStay B (2006) A role for upstream binding factor in organizing ribosomal gene chromatin. Biochem Soc Symp 73:77–84
Bell SP, Learned RM, Jantzen HM, Tjian R (1988) Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 241:1192–1197
Pikaard CS, McStay B, Schultz MC, Bell SP, Reeder RH (1989) The Xenopus ribosomal gene enhancers bind an essential polymerase I transcription factor, xUBF. Genes Dev 3:1779–1788
Pikaard CS, Smith SD, Reeder RH, Rothblum L (1990) rUBF, an RNA polymerase I transcription factor from rats, produces DNase I footprints identical to those produced by xUBF, its homolog from frogs. Mol Cell Biol 10:3810–3812
Moss T, Stefanovsky VY, Pelletier G (1998) The structural and architectural role of upstream binding factor, UBF. In: Paule MR (ed) Transcription of ribosomal RNA genes by eukaryotic RNA polymerase I. Springer, Berlin, pp 75–94
Bazett-Jones DP, Leblanc B, Herfort M, Moss T (1994) Short-range DNA looping by the Xenopus HMG-box transcription factor, xUBF. Science 264:1134–1137
Hamdane N, Stefanovsky VY, Tremblay MG, Németh A, Paquet E, Lessard F, Sanij E, Hannan R, Moss T (2014) Conditional inactivation of Upstream Binding Factor reveals its epigenetic functions and the existence of a somatic nucleolar precursor body. PLoS Genet 10:e1004505
O’Sullivan AC, Sullivan GJ, McStay B (2002) UBF binding in vivo is not restricted to regulatory sequences within the vertebrate ribosomal DNA repeat. Mol Cell Biol 22:657–668
Roussel P, André C, Masson C, Géraud G, Hernandez-Verdun D (1993) Localization of the RNA polymerase I transcription factor hUBF during the cell cycle. J Cell Sci 104(Pt 2):327–337
Jordan P, Mannervik M, Tora L, Carmo-Fonseca M (1996) In vivo evidence that TATA-binding protein/SL1 colocalizes with UBF and RNA polymerase I when rRNA synthesis is either active or inactive. J Cell Biol 133:225–234
Roussel P, André C, Comai L, Hernandez-Verdun D (1996) The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J Cell Biol 133:235–246
Zatsepina OV, Schöfer C, Weipoltshammer K, Mosgoeller W, Almeder M, Stefanova VN, Jordan EG, Wachtler F (1996) The RNA polymerase I transcription factor UBF and rDNA are located at the same major sites in both interphase and mitotic pig embryonic kidney (PK) cells. Cytogenet Cell Genet 73:274–278
Heitz E (1931) Die Ursache der gesetzmässigen Zahl, Lage, Form und Grösse pflanzlicher Nukleolen. Planta 12:775–844
Heliot L, Kaplan H, Lucas L, Klein C, Beorchia A, Doco-Fenzy M, Menager M, Thiry M, O’Donohue MF, Ploton D (1997) Electron tomography of metaphase nucleolar organizer regions: evidence for a twisted-loop organization. Mol Biol Cell 8:2199–2216
McClintock B (1934) The relation of a particular chromosomal element to the development of the nucleoli in Zea mays. Z Für Zellforsch Mikrosk Anat 21:294–326
Albert B, Colleran C, Léger-Silvestre I, Berger AB, Dez C, Normand C, Perez-Fernandez J, McStay B, Gadal O (2013) Structure-function analysis of Hmo1 unveils an ancestral organization of HMG-Box factors involved in ribosomal DNA transcription from yeast to human. Nucleic Acids Res 41:10135–10149
Gadal O, Labarre S, Boschiero C, Thuriaux P (2002) Hmo1, an HMG-box protein, belongs to the yeast ribosomal DNA transcription system. EMBO J 21:5498–5507
Berger AB, Decourty L, Badis G, Nehrbass U, Jacquier A, Gadal O (2007) Hmo1 is required for TOR-dependent regulation of ribosomal protein gene transcription. Mol Cell Biol 27:8015–8026
Hall DB, Wade JT, Struhl K (2006) An HMG protein, Hmo1, associates with promoters of many ribosomal protein genes and throughout the rRNA gene locus in Saccharomyces cerevisiae. Mol Cell Biol 26:3672–3679
Kasahara K, Ohtsuki K, Ki S, Aoyama K, Takahashi H, Kobayashi T, Shirahige K, Kokubo T (2007) Assembly of regulatory factors on rRNA and ribosomal protein genes in Saccharomyces cerevisiae. Mol Cell Biol 27:6686–6705
Kermekchiev M, Workman JL, Pikaard CS (1997) Nucleosome binding by the polymerase I transactivator upstream binding factor displaces linker histone H1. Mol Cell Biol 17:5833–5842
McCauley MJ, Huo R, Becker N, Holte MN, Muthurajan UM, Rouzina I, Luger K, Maher LJ, Israeloff NE, Williams MC (2019) Single and double box HMGB proteins differentially destabilize nucleosomes. Nucleic Acids Res 47:666–678
Murugesapillai D, McCauley MJ, Huo R, Nelson Holte MH, Stepanyants A, Maher LJ, Israeloff NE, Williams MC (2014) DNA bridging and looping by HMO1 provides a mechanism for stabilizing nucleosome-free chromatin. Nucleic Acids Res 42:8996–9004
Hu CH, McStay B, Jeong SW, Reeder RH (1994) xUBF, an RNA polymerase I transcription factor, binds crossover DNA with low sequence specificity. Mol Cell Biol 14:2871–2882
Kamau E, Bauerle KT, Grove A (2004) The Saccharomyces cerevisiae high mobility group box protein HMO1 contains two functional DNA binding domains. J Biol Chem 279:55234–55240
Leblanc B, Read C, Moss T (1993) Recognition of the Xenopus ribosomal core promoter by the transcription factor xUBF involves multiple HMG box domains and leads to an xUBF interdomain interaction. EMBO J 12:513–525
Sanij E, Diesch J, Lesmana A, Poortinga G, Hein N, Lidgerwood G, Cameron DP, Ellul J, Goodall GJ, Wong LH, Dhillon AS, Hamdane N, Rothblum LI, Pearson RB, Haviv I, Moss T, Hannan RD (2015) A novel role for the Pol I transcription factor UBTF in maintaining genome stability through the regulation of highly transcribed Pol II genes. Genome Res 25:201–212
Achar YJ, Adhil M, Choudhary R, Gilbert N, Foiani M (2020) Negative supercoil at gene boundaries modulates gene topology. Nature 577:701–705
Mais C, Wright JE, Prieto J-L, Raggett SL, McStay B (2005) UBF-binding site arrays form pseudo-NORs and sequester the RNA polymerase I transcription machinery. Genes Dev 19:50–64
Prieto J-L, McStay B (2008) Pseudo-NORs: a novel model for studying nucleoli. Biochim Biophys Acta 1783:2116–2123
Prieto J-L, McStay B (2007) Recruitment of factors linking transcription and processing of pre-rRNA to NOR chromatin is UBF-dependent and occurs independent of transcription in human cells. Genes Dev 21:2041–2054
Lucchini R, Sogo JM (1995) Replication of transcriptionally active chromatin. Nature 374:276–280
Scott RS, Truong KY, Vos JM (1997) Replication initiation and elongation fork rates within a differentially expressed human multicopy locus in early S phase. Nucleic Acids Res 25:4505–4512
Sandmeier JJ, French S, Osheim Y, Cheung WL, Gallo CM, Beyer AL, Smith JS (2002) RPD3 is required for the inactivation of yeast ribosomal DNA genes in stationary phase. EMBO J 21:4959–4968
Fahy D, Conconi A, Smerdon MJ (2005) Rapid changes in transcription and chromatin structure of ribosomal genes in yeast during growth phase transitions. Exp Cell Res 305:365–373
Tremblay M, Charton R, Wittner M, Levasseur G, Griesenbeck J, Conconi A (2014) UV light-induced DNA lesions cause dissociation of yeast RNA polymerases-I and establishment of a specialized chromatin structure at rRNA genes. Nucleic Acids Res 42:380–395
Conconi A, Paquette M, Fahy D, Bespalov VA, Smerdon MJ (2005) Repair-independent chromatin assembly onto active ribosomal genes in yeast after UV irradiation. Mol Cell Biol 25:9773–9783
Sanij E, Poortinga G, Sharkey K, Hung S, Holloway TP, Quin J, Robb E, Wong LH, Thomas WG, Stefanovsky V, Moss T, Rothblum L, Hannan KM, McArthur GA, Pearson RB, Hannan RD (2008) UBF levels determine the number of active ribosomal RNA genes in mammals. J Cell Biol 183:1259–1274
Birch JL, Tan BC-M, Panov KI, Panova TB, Andersen JS, Owen-Hughes TA, Russell J, Lee S-C, Zomerdijk JCBM (2009) FACT facilitates chromatin transcription by RNA polymerases I and III. EMBO J 28:854–865
Cutler S, Lee LJ, Tsukiyama T (2018) Chromatin remodeling factors Isw2 and Ino80 regulate chromatin, replication, and copy number of the Saccharomyces cerevisiae ribosomal DNA locus. Genetics 210:1543–1556
Hamperl S, Brown CR, Garea AV, Perez-Fernandez J, Bruckmann A, Huber K, Wittner M, Babl V, Stoeckl U, Deutzmann R, Boeger H, Tschochner H, Milkereit P, Griesenbeck J (2014) Compositional and structural analysis of selected chromosomal domains from Saccharomyces cerevisiae. Nucleic Acids Res 42:e2
Zhang Y, Anderson SJ, French SL, Sikes ML, Viktorovskaya OV, Huband J, Holcomb K, Hartman JL, Beyer AL, Schneider DA (2013) The SWI/SNF chromatin remodeling complex influences transcription by RNA polymerase I in Saccharomyces cerevisiae. PloS One 8:e56793
Anderson SJ, Sikes ML, Zhang Y, French SL, Salgia S, Beyer AL, Nomura M, Schneider DA (2011) The transcription elongation factor Spt5 influences transcription by RNA polymerase I positively and negatively. J Biol Chem 286:18816–18824
Engel KL, French SL, Viktorovskaya OV, Beyer AL, Schneider DA (2015) Spt6 is essential for rRNA synthesis by RNA polymerase I. Mol Cell Biol 35:2321–2331
Iben S, Tschochner H, Bier M, Hoogstraten D, Hozák P, Egly JM, Grummt I (2002) TFIIH plays an essential role in RNA polymerase I transcription. Cell 109:297–306
Schneider DA, French SL, Osheim YN, Bailey AO, Vu L, Dodd J, Yates JR, Beyer AL, Nomura M (2006) RNA polymerase II elongation factors Spt4p and Spt5p play roles in transcription elongation by RNA polymerase I and rRNA processing. Proc Natl Acad Sci U S A 103:12707–12712
Zhang Y, Sikes ML, Beyer AL, Schneider DA (2009) The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc Natl Acad Sci U S A 106:2153–2158
Zhang Y, French SL, Beyer AL, Schneider DA (2016) The transcription factor THO promotes transcription initiation and elongation by RNA polymerase I. J Biol Chem 291:3010–3018
Gallagher JEG, Dunbar DA, Granneman S, Mitchell BM, Osheim Y, Beyer AL, Baserga SJ (2004) RNA polymerase I transcription and pre-rRNA processing are linked by specific SSU processome components. Genes Dev 18:2506–2517
Merkl PE, Pilsl M, Fremter T, Schwank K, Engel C, Längst G, Milkereit P, Griesenbeck J, Tschochner H (2020) RNA polymerase I (Pol I) passage through nucleosomes depends on Pol I subunits binding its lobe structure. J Biol Chem 295:4782–4795
Hermans N, Huisman JJ, Brouwer TB, Schächner C, van Heusden GPH, Griesenbeck J, van Noort J (2017) Toehold-enhanced LNA probes for selective pull down and single-molecule analysis of native chromatin. Sci Rep 7:16721
Acknowledgments
We are grateful to all scientists who contributed to this exciting field of research and apologize to those colleagues whose work has not been cited. We thank the members of the department of Biochemistry III for constant support and discussion. This work was funded by the Deutsche Forschungsgemeinschaft (DFG) in the context of the SFB960. P. E. M. was partly supported by a fellowship of the German National Academic Foundation.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Schächner, C. et al. (2022). Establishment and Maintenance of Open Ribosomal RNA Gene Chromatin States in Eukaryotes. In: Entian, KD. (eds) Ribosome Biogenesis. Methods in Molecular Biology, vol 2533. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2501-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2501-9_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2500-2
Online ISBN: 978-1-0716-2501-9
eBook Packages: Springer Protocols