Abstract
Immunoglobulin (IG) clonality assessment is a widely used supplementary test for the diagnosis of suspected lymphoid malignancies. The specific rearrangements of the immunoglobulin (IG) heavy and light chain genes act as a unique hallmark of a B-cell lymphoma, a feature that is used in clonality assessment. The widely used BIOMED-2/EuroClonality IG clonality assay, visualized by GeneScanning or heteroduplex analysis, has an unprecedented high detection rate because of the complementarity of this approach. However, the BIOMED-2/EuroClonality clonality assays have been developed for the assessment of specimens with optimal DNA quality. Further improvements for the assessment of samples with suboptimal DNA quality, such as from formalin-fixed paraffin-embedded (FFPE) specimens or specimens with a limited tumor burden, are required. The EuroClonality-NGS Working Group recently developed a next-generation sequencing (NGS)-based clonality assay for the detection of the IG heavy and kappa light chain rearrangements, using the same complementary approach as in the conventional assay. By employing next-generation sequencing, both the sensitivity and specificity of the clonality assay have increased, which not only is very useful for diagnostic clonality testing but also allows robust comparison of clonality patterns in a patient with multiple lymphoma’s that have suboptimal DNA quality. Here, we describe the protocols for IG-NGS clonality assessment that are compatible for Ion Torrent and Illumina sequencing platforms including pre-analytical DNA isolation, the analytical phase, and the post-analytical data analysis.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- Clonality analysis
- Next-generation sequencing
- B-cell lymphoma
- Immunoglobulin gene rearrangements
- ARResT/Interrogate
1 Introduction
Clonality assessment of the immunoglobulin (IG) or T-cell receptor genes is a useful supplementary tool for the diagnosis of B-cell and T-cell lymphoid malignancies. Cancer cells have a unique feature that they originate from a single transformed cell. The malignant cells of a B-cell lymphoma all have the same rearranged IG DNA sequences encoding for a unique antigen-receptor molecule, also called the B-cell receptor (BCR ). Clonality assessment makes use of this feature. In patients suspected for having a B-cell lymphoma, clonality assessment enables demonstration of a clonal expansion of clonally related B cells, all having the identical molecular footprint of the antigen receptor encoded by the IG genes.
1.1 Immunoglobulin Gene Rearrangements
The BCR consists of two IG heavy chains (IGH ) and two light chains, IG kappa (IGK ) or IG lambda (IGL ), with unique nucleotide sequences at the antigen binding region that are generated during lymphoid development. The proper assembly of a functional BCR is controlled by several checkpoints at different stages of B-cell development [1,2,3]. Once a mature B cell has encountered an antigen, it will undergo somatic hypermutation (SHM) in the germinal center. During this process that is mediated by the enzyme activation-induced cytidine deaminase (AID), random sequence alterations [mostly point mutations, but deletions or insertions can occur as well] are introduced to improve antigen binding, a phenomenon called affinity maturation [2, 3].
The BCR is generated by a stepwise process involving rearrangements of the different germline variable (V), diversity (D), and joining (J) IG genes, called V(D)J recombination. This process is initiated by the recombination-activating gene (RAG) products RAG1 and RAG2 [4, 5], which relies on the recognition of recombination signal sequences (RSSs) flanking the individual genes. V(D)J recombination starts with the IG heavy chain, by the recombination of one of the D genes with one of the J genes, followed by the subsequent joining of one of the V genes to the rearranged DJ gene (Fig. 1). This random recombination of V, D, and J genes generates the so-called combinatorial diversity. Imprecise joining of the genes by the activation of exonucleases, as well as the addition of non-template DNA nucleotides by the enzyme terminal deoxynucleotidyl transferase (TdT), results in junctional diversity, on top of the combinatorial diversity. As a consequence of the combinatorial and junctional diversity, only one out of three VDJ rearrangements will be able to express a functional BCR . This high frequency of out-of-frame rearrangements may explain why many of the B lymphocytes have rearranged both their IGH genes, so-called biallelic IGH gene rearrangements. Lymphomas with biallelic gene rearrangements occur frequently, whereas lymphomas that are truly bi-clonal are rare [7].
Detection of V(D)J gene rearrangements at the immunoglobulin heavy chain locus. After a functional DJ rearrangement has been generated, a V gene is joined to this DJ fragment. Each B cell may generate one (productive rearrangement) or two (an unproductive and productive rearrangement) specific clonotypes that consist of one IGHV, IGHD, and IGHJ gene segment. The locations of the primers used for IG-NGS clonality assessment are indicated by arrows. For detection of IGHV-IGHD-IGHJ gene rearrangements, the forward primers are located in framework region 3 (VH FR3), which are combined with IGHJ reverse primers. The detection of unproductive, incomplete IGHD-IGHJ rearrangements makes use of forward IGHD primers (located 5′ of the IGHD genes) and reverse IGHJ primers, hence enabling detection of incompletely rearranged IGHD-IDHJ joining. Once an IGHV gene is recombined to the IGHD-IGHJ segment, the IGHD primer binding site will be removed. Successful amplification will result in DNA fragments that cover the junctional region with a specific amino acid length. Figure adapted from Scheijen et al., 2019 [6]
For the light chain (IGK or IGL ), a direct V to J gene rearrangement takes place, where the IGK locus will first undergo gene rearrangement. When there is no productive IGKV-IGKJ rearrangement, additional rearrangements will occur that inactivate the IGK locus by removal of the IGKC region and the enhancers. These rearrangements involve the KDE sequence that can rearrange to one of the kappa V genes and thereby delete the initial IGKV-IGKJ rearrangement, resulting in an IGKV-KDE rearrangement or to an isolated recombination signal sequence (RSS) that is located in the J kappa-C kappa intron (intron RSS), resulting in an Intron RSS-KDE rearrangement [8] (Fig. 2). If there is no proper IGK rearrangement, the IGL genes will rearrange. Theoretically, all mature B-cell malignancies should possess IGK rearrangements, regardless of the light chain expression [9]. Based on the amount of functional genes, the estimated number of unique BCRs generated by combinational diversity of both the heavy and light chain is 4.6 × 106 [10]. However, the actual number of unique receptors is lower, since not all genes are used at the same frequency, and not every heavy and light chain can pair to form a functional BCR .
IGK rearrangements involving Kappa deleting element. IGK gene rearrangement starts with an initial IGKV-IGKJ recombination. If this results in a productive rearrangement, no subsequent recombination events will occur within the IGK locus. However, in case there is an unproductive IGKV-IGKJ rearrangement, this may lead to inactivation of the IGK locus involving rearrangements with the Kappa deleting element (KDE) sequence. This can include a rearrangement between KDE and Intron RSS-KDE recombination on the same allele (Allele A). The initially formed unproductive IGKV-IGKJ segment remains present on that allele. Both the unproductive IGKV-IGKJ and Intron RSS-KDE rearrangements are detectable with clonality analysis. The second option, involves a recombination of an upstream IGKV gene with the KDE sequence, thereby deleting the preexisting unproductive IGKV-IGKJ rearrangement on that allele (Allele B). Potentially, up to four distinct IGK rearrangements can be generated that go along in one B cell clone. The locations of the primers used for IG-NGS clonality assessment are indicated by arrows. Figure adapted from Scheijen et al., 2019 [6]
The junctional diversity further increases BCR diversity by a factor 10.
B cells that assembled a functional BCR will further diversify by undergoing somatic SHM to extend the IG repertoire upon antigen recognition within the germinal center of a lymph node [2, 11]. When B cells fail or become autoreactive during this process, they will be silenced and eliminated [1, 3].
1.2 Clonality Detection in B-Cell Lymphoma Based on BIOMED-2/EuroClonality Assays
Clonality assessment by detecting IG gene rearrangements is widely used for diagnostics, and multiple assays have been developed over the years, which differ in the level of sensitivity [12]. The current gold standard are the PCR-based BIOMED-2/EuroClonality assays, visualized with either GeneScan fragment analysis or heteroduplex analysis [13, 14]. In this assay, standardized PCR protocols are used that cover IGH and IGK gene rearrangements. These include complete IGHV-IGHD-IGHJ rearrangements but also incomplete IGHD-IGHJ rearrangements, which are not affected by somatic hypermutation either. For IGK gene rearrangements, not only IGKV-IGKJ rearrangements are included but also rearrangements involving KDE, which are not affected by somatic hypermutation. Notably, these occur on one or both alleles in virtually all IgLambda-positive B-cell malignancies and in one-third of the IgKappa-positive B-cell malignancies. The primers and protocols of the BIOMED-2/EuroClonality PCR assays allow detection of virtually all clonal B-cell proliferations, and the primer design has been based on family primers and consensus primers relevant for the IG genes. A clonal cell population gives rise to one or two dominant PCR products of a given size on GeneScan. A polyclonal cell population will result in a range of differently sized PCR fragments, corresponding to the presence of different V(D)J gene rearrangements showing Gaussian distribution with respect to the amount of inserted or deleted nucleotides in the junctional region.
The BIOMED-2/EuroClonality assays are used worldwide and have resulted in increased clonality detection of lymphoid malignancies [15, 16]. However, there are still some drawbacks that could potentially yield (mainly) false-negative results. The BIOMED-2/EuroClonality assays have been designed for high-quality DNA samples generating amplicons in the range of 150–400 bp. However, formalin-fixed paraffin-embedded (FFPE) tissue specimens, which are mostly used in a diagnostic setting, may yield DNA samples of inferior quality. Clonal rearrangements that correspond to relatively longer amplicons may therefore potentially be missed [13, 15, 17]. Furthermore, detection of minor clones in a background of nonmalignant B cells is highly dependent on the position of the clonal product within the Gaussian curve of the polyclonal background, where it can be difficult or even impossible to detect these minor clones.
1.3 NGS-Based Clonality Detection in B-Cell Lymphomas
To further improve the application potential of clonality assessment, the EuroClonality-NGS Working Group has developed a novel next-generation sequencing (NGS)-based clonality assay for detection of IG gene rearrangements (IG-NGS) [6], together with the bioinformatics tool ARResT/Interrogate [18]. New primers were designed for the incomplete and complete IGH gene rearrangements, the complete IGK rearrangements as well as for the IGK rearrangements involving KDE, again making use of the complementary approach that is one of the strengths of the conventional BIOMED-2/EuroClonality assays. The primer design for the NGS-based clonality assay is based on gene-specific primers for the relevant genes and, importantly, on the generation of shorter amplicon sizes, which makes it more suitable for clonality detection in samples of inferior DNA quality. Furthermore, the IG-NGS assay immediately provides the nucleotide sequences of the identified clonotypes from both the malignant clone and the nonmalignant background B cells. Using this sequence information, reliable detection of minor clones is possible, resulting in a high sensitivity of the clonality analysis as recently described by Scheijen et al. [6]. Clonal rearrangements of lymphomas with a high tumor load still can be traced back when diluted in a concentration of 5% and 2.5% in a polyclonal background of tonsil DNA. The detection rate of 2.5% is not possible by the conventional assay combined with GeneScanning or heteroduplex, because the clonal product will be blurred by the polyclonal background [13]. Furthermore, the sequence information, the design for suboptimal DNA specimen, and the sensitivity are extremely valuable for comparison of sequential lesions or multiple lymphomas at different locations in a single patient.
1.4 Different NGS Platforms for Clonality Testing: Ion Torrent Versus Illumina
Similar to the BIOMED-2 approach, the IG-NGS clonality assay is based on a multiplex PCR to amplify the target regions and by subsequent ligation of adaptors for sequencing. The targets detected in the NGS clonality assay include IGH (IGHV-IGHD-IGHJ and IGHD-IGHJ) and IGK (IGKV-IGKJ, IGKV-KDE, and Intron RSS-KDE) gene rearrangements. After purification of the PCR products, the library preparation is performed, followed by sequencing on Ion Torrent or Illumina platforms (Fig. 3).
Schematic workflow for IG-NGS clonality assay. A multiplex PCR is performed on extracted DNA of specimens suspect for lymphoproliferations to amplify IGHV-IGHD-IGHJ, IGHD-IGHJ, IGKV-IGKJ, and IGKV/Intron RSS-KDE gene rearrangements. Library preparation for sequencing on Ion Torrent (left panel) or Illumina (right panel) is shown. The Ion Torrent library preparation is an adapter ligation protocol, requiring end repair of the obtained amplicons and the ligation of barcode and adapters to them and nick repair , followed by a final library amplification step. Library preparation for Illumina is a two-step PCR protocol in which the target-specific amplicons are generated using primers containing an M13 adapter, which is used in the second PCR to add specific barcodes to them. Obtained sequencing data is analyzed using the bio-informatics tool ARResT/Interrogate
The initial IG-NGS workflow described the protocols for the Ion Torrent platform [6], a technique that makes use of electrochemical detection of hydrogen ions that are released during DNA synthesis [19]. The Illumina platform represents also a widely used NGS application in diagnostic laboratories, and both are very suitable for high-throughput NGS-based molecular assays. In contrast to Ion Torrent-based sequencing, Illumina employs fluorescently labeled nucleotides that are incorporated during complementary DNA strand synthesis [20]. Depending on the type of Illumina sequencer, this can be a 2-channel (e.g., MiniSeq, NextSeq, NovaSeq) or 4-channel chemistry (e.g., MiSeq, HiSeq).
The Ion Torrent and the Illumina sequencing technologies require specific adapters for sequencing and barcodes for sample identification. In the workflow that was developed for Ion Torrent sequencing, the adapters and barcodes are ligated to the amplicons (adapter ligation protocol). For Illumina sequencing platforms, the sequencing adapters need to be incorporated in the amplicon primers. Recently, the EuroClonality-NGS Working Group described a two-step PCR assay for minimal residual disease (MRD) target identification using an Illumina-compatible workflow [21]. With this approach, the barcoded adapter sequences are incorporated in the second PCR of the two-step PCR assay with universal barcoded M13-tailed primers. The workflow for clonality detection using the Illumina sequencing platform that will be described in this chapter is based on this previously described two-step PCR protocol [21], with some minor modifications in the first PCR reaction and purification steps of the amplicons as well as the PCR conditions of the Ion Torrent protocol (Table 1) (see Note 1).
In the subsequent paragraphs, we present a complete overview of the different steps of IG-NGS clonality analysis in suspected B-cell malignancies that are compatible for either Ion Torrent or Illumina sequencing platforms. For complete IGH rearrangements, in this NGS approach, framework-3 (FR3) primers are used in contrast to the BIOMED-2/EuroClonality assay that employs additional FR1 and FR2 primers, generating larger-sized products. Data analysis with ARResT/Interrogate and the technical interpretation and reporting of the obtained results will be addressed. It is of utmost importance that molecular clonality results are eventually interpreted in the context of available clinical, morphological, and immunophenotypic data. Also detailed knowledge of the immunobiology of IG gene rearrangements is mandatory to be able to correctly interpret the different molecular patterns.
2 Materials
2.1 General Materials and Equipment
-
1.
Volume-adjustable single-channel and multichannel pipettes.
-
2.
Filter tips.
-
3.
Eppendorf tubes (0.5 ml, 1.5 ml, and 2 ml).
-
4.
0.2 ml PCR tubes/strips (and caps).
-
5.
Vortex.
-
6.
Centrifuge, e.g., Eppendorf Centrifuge 5420 or equivalent equipment.
-
7.
Microcentrifuge, e.g., MiniStar/MiniStar blueline with tube and PCR rotors (VWR).
-
8.
Ethanol 99%, absolute pro analyse (molecular biology quality grade).
-
9.
Low TE-buffer (T10E0.1): 10 mM Tris-HCl pH 8.0 and 0.1 mM EDTA.
-
10.
Nuclease-free water/Milli-Q (MQ).
-
11.
Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific).
-
12.
Qubit Assay tubes (Thermo Fisher Scientific).
-
13.
Qubit Fluorometer (Thermo Fisher Scientific).
-
14.
Thermal cyclers, e.g., Veriti 96-Well Thermal Cycler (Thermo Fisher Scientific), PTC-0200 (MJ Research) or equivalent equipment.
2.2 DNA Isolation
-
1.
Xylene (molecular biology quality grade).
-
2.
TET lysis buffer: 10 mM Tris/HCl pH 8.5, 1 mM EDTA pH 8.0, 0.01% Tween-20.
-
3.
Chelex-100 (Bio-Rad).
-
4.
TSE: 10 mM Tris–HCl pH 7.5, 0.4 M NaCl, 2 mM EDTA pH 8.0.
-
5.
SDS 20%.
-
6.
Proteinase K (QIAGEN).
-
7.
Genomic DNA isolation kit, e.g., QIAamp DNA FFPE Tissue Kit (QIAGEN), QIAamp DNA Micro Kit (QIAGEN) (see Note 2).
2.3 IG-NGS Clonality Assays
2.3.1 Target Amplification and Purifications
-
1.
dNTPs.
-
2.
AmpliTaq Gold DNA Polymerase, kit with GeneAmp 10× Gold Buffer and MgCl2 (Applied Biosystems).
-
3.
Standard purified primers to be dissolved in Low TE-buffer at 300 pmol/μl (300 μM) (e.g., from Sigma-Aldrich; see Tables 2, 3, and 4 and Note 3).
-
4.
DNA LoBind, Deepwell plate 96/500 μl, white border (Eppendorf).
-
5.
Agencourt AMPure XP Beads (Beckman Coulter).
-
6.
Dyna Mag-96side Magnet (Thermo Fisher Scientific).
2.3.2 Ion Torrent Library Preparation and Sequencing
-
1.
Ion Plus Fragment Library kit (Life Technologies).
-
2.
Ion Xpress Barcode Adapters kit (Life Technologies).
-
3.
Ion 318™ Chip Kit v2 BC (Thermo Fisher Scientific) or Ion 520™ Chip Kit (Thermo Fisher Scientific).
-
4.
Ion Torrent template preparation (Ion One Touch system, Ion Chef system) and sequencing equipment (Ion PGM, Ion GeneStudio S5).
2.3.3 Illumina Library Preparation and Sequencing
-
1.
dNTPs.
-
2.
HPLC purified Illumina M13-barcoded primers to be dissolved in Low TE-buffer at 100 pmol/μl (100 μM stock solution, dilute to 5 μM work solution) (e.g., from Sigma-Aldrich; see Table 5).
-
3.
FastStart High Fidelity PCR system, dNTPack (Roche).
-
4.
Sequencing equipment and associated Illumina Reagent Kit (e.g., MiniSeq sequencer and MiniSeq Mid Output Kit).
3 Methods
3.1 Samples and Quality Controls
IG-NGS clonality analysis can be performed on DNA extracted from any preserved human lymphoid tissue. However, each sample type requires a specific extraction procedure for DNA isolation. We here describe DNA extraction methods for formalin-fixed paraffin-embedded (FFPE) and fresh frozen tissue using the Chelex method (FFPE), TSE (fresh frozen), and column-based extraction procedure of QIAGEN; equivalent isolation systems are also possible (see Note 2).
To perform reliable clonality assessment it is important to determine whether a representative tissue section is used, whether obtained DNA is of sufficient quality (see Note 4) and using a standardized DNA input per PCR. Furthermore, robust performance of the multiplex PCR reaction should be assessed by including control samples such as a polyclonal control sample (e.g., tonsil or mononuclear peripheral blood cells) and negative control (water), while preparing the samples for IG-NGS clonality assessment (see Note 5).
3.2 DNA Isolation
For isolation of genomic DNA from FFPE tissue, different methods are available. Here two of such protocols are described, a commercially available DNA isolation kit (QIAGEN) and the Chelex method. Both protocols use a microcolumn purification of the extracted DNA; this is an important step in preparing DNA samples for clonality assays and is described in Subheading 3.2.3. Finally, a protocol for isolation of genomic DNA from fresh frozen tissue is described.
3.2.1 DNA Extraction from Formalin-Fixed Paraffin-Embedded Tissue with Genomic DNA Isolation Kit
All steps are performed at room temperature, unless specified otherwise.
-
1.
Place two to five 10 μm sections of FFPE tissue (of approximately 1 cm2 in size) into a 1.5 ml Eppendorf tube (see Note 4).
-
2.
Add 1000 μl xylene and vortex thoroughly for 10 s (work in a protective cabinet) (see Note 6).
-
3.
Centrifuge for 5 min, 20,000 × g.
-
4.
Remove the supernatant carefully and dispense it in specific waste containers.
-
5.
Add 1000 μl 99% ethanol.
-
6.
Centrifuge for 5 min, 20,000 × g.
-
7.
Remove carefully the ethanol.
-
8.
Add again 1000 μl 99% ethanol and mix carefully by inverting the tube.
-
9.
Centrifuge for 5 min, 20,000 × g.
-
10.
Remove all ethanol and air-dry the remaining tissue by leaving the tube open, and incubate for 10–15 min at 37 °C in a thermo block.
-
11.
Resuspend the pellet in 180 μl Buffer ATL.
-
12.
Add 20 μl proteinase K, and mix by vortexing.
-
13.
Incubate overnight at 56 °C (see Note 7).
-
14.
Incubate at 90 °C for 1 h (see Note 8).
-
15.
Add 200 μl Buffer AL to the sample, and mix thoroughly by vortexing (see Note 9).
-
16.
Add 200 μl ethanol (96–100%), and mix again thoroughly by vortexing (see Note 9).
-
17.
Continue with the extraction and DNA purification as described in Subheading 3.2.3, before starting the sample preparations for clonality assessment.
3.2.2 DNA Extraction from Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Starting with the Chelex Method
This Chelex-based DNA extraction protocol is developed as common workflow that is suitable for the majority of the molecular tests used in diagnostics. However, for clonality assessment, it is important to purify the DNA obtained with this protocol before use in the clonality assay in order to obtain good quality results.
All steps are performed at room temperature, unless specified otherwise.
-
1.
Deparaffinize two to five 10 μm tissue sections as described in Subheading 3.2.1 until step 10.
-
2.
Add 200 μl of 5% Chelex-100 homogeneously mixed in TET lysis buffer.
-
3.
Incubate for 5 min at 95 °C in a thermo shaker at 350 rpm.
-
4.
Cool down for 5 min at room temperature.
-
5.
Add 20 μl of proteinase K and incubate o/n at 56 °C in a thermo shaker at 350 rpm (see Note 7).
-
6.
Incubate for 10 min at 95 °C in a thermo shaker at 350 rpm (see Note 8).
-
7.
Centrifuge for 10 min, 20,000 × g at room temperature.
-
8.
Transfer the supernatant to a clean 1.5 ml Eppendorf tube.
-
9.
Centrifuge for 10 min, 20,000 × g at room temperature.
-
10.
Transfer the supernatant to a clean 1.5 ml Eppendorf tube.
-
11.
Add 180 μl ATL buffer (QIAamp DNA Micro Kit), vortex for 10 s, and incubate at room temperature for 30 min.
-
12.
Incubate at 80 °C for 10 min, and then allow to cool down to room temperature.
-
13.
Briefly centrifuge the 1.5 ml tube to remove drops from the inside of the lid.
-
14.
Add 200 μl buffer AL and vortex briefly.
-
15.
Incubate at 70 °C for 10 min, and then allow to cool down to room temperature.
-
16.
Add 250 μl of ethanol 96%, vortex briefly.
-
17.
Continue the procedure with the DNA purification as described in Subheading 3.2.3, before starting the sample preparations for clonality assessment.
3.2.3 DNA Purification with QIAamp DNA Microcolumn
All steps are performed at room temperature.
-
1.
Carefully transfer the entire lysate to the QIAamp MinElute column (in a 2 ml collection tube) and centrifuge at 6000 × g for 1 min (see Note 10).
-
2.
Place the QIAamp MinElute column in a clean 2 ml collection tube, and discard the collection tube containing the flow-through.
-
3.
Add 500 μl Buffer AW1 on the column and centrifuge at 6000 × g for 1 min (see Note 11).
-
4.
Discard the flow-through and add 500 μl Buffer AW2 to the column (see Note 11).
-
5.
Centrifuge at 6000 × g for 1 min and discard the flow-through.
-
6.
Centrifuge at full speed (20,000 × g) for 3 min to dry the membrane completely (see Note 12).
-
7.
Place the QIAamp MinElute column in a clean 1.5 ml Eppendorf tube, and discard the collection tube containing the flow-through.
-
8.
Apply 20–100 μl Buffer ATE (QIAamp DNA FFPE Kit) or 20–100 μl Buffer AE (QIAamp DNA Micro Kit) to the center of the column membrane (see Note 13).
-
9.
Incubate at room temperature for 5 min.
-
10.
Centrifuge at full speed (20,000 × g) for 1 min.
-
11.
Discard the column and keep the 1.5 ml tube containing the DNA solution.
-
12.
Determine the DNA concentration and dilute if necessary to a working solution of 20–40 ng/μl with the used elution buffer or MQ (see Note 14).
3.2.4 DNA Extraction from Fresh Frozen Tissue: TSE Method
All steps are performed at room temperature, unless specified otherwise.
-
1.
Place five to ten 10 μm tissue sections in a 1.5 ml tube with 400 μl TSE (see Note 15).
-
2.
Add 21 μl SDS 20% and 37.5 μl proteinase K (20 mg/ml) and mix manually.
-
3.
Incubate o/n at 56 °C on a thermo shaker at 350 rpm (see Note 7).
-
4.
Keep the lysate in the 1.5 ml Eppendorf tube.
-
5.
Add 168 μl NaCl 5 M and shake the tube for 15 s.
-
6.
Centrifuge for 15 min at 18,000 × g.
-
7.
Transfer the supernatant to a clean 2 ml Eppendorf tube.
-
8.
Centrifuge for 15 min at 18,000 × g.
-
9.
Transfer the supernatant to a clean 2 ml Eppendorf tube.
-
10.
Add 1.2 ml ethanol 100% and turn around the tube a few times so the DNA will precipitate (see Note 16).
-
11.
Centrifuge for 10 min at 18,000 × g.
-
12.
Remove the supernatant carefully using a pipette.
-
13.
Wash the pellet with 1 ml ethanol 70%.
-
14.
Centrifuge for 10 min at 18,000 × g.
-
15.
Remove the supernatant carefully using a pipette.
-
16.
Air-dry the pellet for at least 1 min until the pellet is completely dry.
-
17.
Add Low TE-buffer to the pellet (20–50 μl when there is not a clearly visible pellet present; use larger volumes when the DNA pellet is bigger).
-
18.
Incubate for at least 2 h at room temperature on a roller bank or incubate for longer time (o/n or longer) at 4 °C to allow the DNA to dissolve completely.
-
19.
Determine the DNA concentration and dilute if necessary to a working solution of 20–40 ng/μl with Low TE-buffer or MQ (see Note 14).
3.3 Ion Torrent Protocol for IG-NGS Clonality Assessment
3.3.1 Multiplex PCR for Amplification of IGH-FR3, IGHD, and IGK
-
1.
Prepare three different 0.2 ml PCR tubes per sample: IGH-FR3, IGHD, and IGK (see Table 1, panel “Ion Torrent protocol for clonality detection”).
-
2.
Add 40 ng DNA (Qubit measured; see Note 14) and the other components of the reaction, according to Table 1 (panel “Ion Torrent protocol for clonality detection”), and adjust the total PCR volume to 25 μl with MQ (see Note 17).
-
3.
Use a pipette to mix the prepared PCR reaction with sample thoroughly while avoiding air bubbles in the reaction mix and perform a quick spin to collect all liquid to the bottom of the PCR tube.
-
4.
Run the PCR in a thermocycler with heated lid, according to the program in Table 1 (panel “Ion Torrent protocol for clonality detection”).
-
5.
After completing the PCRs, combine the tree targets IGH-FR3, IGHD, and IGK per sample (~75 μl total volume).
3.3.2 Cleanup of IGH-FR3, IGHD, and IGK Amplicons
-
1.
Allow the AMPure XP magnetic beads to warm to room temperature for at least 30 min before use. Ensure that the beads are homogeneous prior to use by mixing the tube by hand for 20 s (see Note 18).
-
2.
Pipette the pooled samples in a DNA LoBind plate, and add 1.8 times (135 μl) volume Agencourt AMPure XP magnetic beads per sample (see Note 19).
-
3.
Use a pipette to mix the solution thoroughly (avoid air bubbles), until the beads and sample are homogeneously mixed, and incubate for 5 min at room temperature.
-
4.
Place the samples for 2–5 min in a magnetic stand until the solution is clear (see Note 20).
-
5.
Carefully remove the supernatant using a 200 μl pipette (see Note 21).
-
6.
Add 150 μl freshly made 70% ethanol per sample (see Note 22).
-
7.
Move the plate in the magnetic stand approximately 4 times from left to right, and make sure the bead pellet migrates and is washed clean.
-
8.
Carefully remove the supernatant using a 200 μl pipette (see Note 21).
-
9.
Repeat steps 6–8 once.
-
10.
Carefully remove any remaining supernatant using a 10 μl pipette, and air-dry the beads for 5 min to allow complete evaporation of residual ethanol (see Note 23).
-
11.
Resuspend the samples in 25 μl Low TE-buffer.
-
12.
Use a pipette to mix the solution thoroughly (avoid air bubbles), to generate a homogeneously mixed solution, and incubate for 2 min at room temperature.
-
13.
Place the samples for 2 min in the magnetic stand until the solution is clear (see Note 20).
-
14.
Collect the purified DNA by pipetting the solution (~25 μl) into a new PCR strip (see Note 24).
3.3.3 End Repair of Amplicons
-
1.
Measure the DNA concentration of every individual sample using Fluorometric Quantitation (using 2 μl of the sample for the Qubit high sensitivity assay).
-
2.
Transfer max. 40 ng DNA to a 0.2 ml PCR tube for the end repair step. In case the total yield is less than 40 ng, use as much as possible. Adjust the volume to 39.5 μl with Low TE-buffer.
-
3.
Add the reagents for the end repair reaction from the Ion Plus Fragment Library kit to the amplicons according to Table 6 (see Note 25).
-
4.
Use a pipette to mix the suspension thoroughly, perform a quick spin to collect all liquid to the bottom, and incubate 30 min at room temperature (see Note 26).
-
5.
Perform a cleanup as described in Subheading 3.3.2 with 1.8 times volume Agencourt AMPure XP magnetic beads (90 μl) and elution volume of 25 μl Low TE-buffer.
3.3.4 Adapter Ligation
-
1.
To ligate adapters to the amplicon and to perform nick repair, for each sample, add the amplicons and reagents from the Ion Plus Fragment Library kit and Ion Xpress Barcode Adapter kit to a 0.2 ml PCR tube according to Table 7 (see Note 27). Make sure that for each sample a different barcode is used (see Note 28).
-
2.
Run the adapter ligation program according to Table 8.
-
3.
Perform a cleanup as in Subheading 3.3.2 with 1.8 times volume Agencourt AMPure XP magnetic beads (90 μl) and elution volume of 13 μl Low TE-buffer (see Note 29).
3.3.5 Library Amplification
-
1.
To amplify the libraries, for each sample, add the purified adapter-ligated amplicons (see Note 29) and reagents from the Ion Plus Fragment Library kit to a 0.2 ml PCR tube according to Table 9.
-
2.
Use a pipette to mix the suspension thoroughly and perform a quick spin to collect all liquid from the sides of the tube.
-
3.
Run the PCR in a thermocycler with heated lid, according to the program in Table 10.
-
4.
Perform a cleanup as in Subheading 3.3.2 with 1.4 times volume Agencourt AMPure XP magnetic beads per sample (90 μl) and elution volume of 25 μl Low TE-buffer.
3.3.6 Ion Torrent Sequencing Run
-
1.
Measure the DNA concentration of all samples using Fluorometric Quantitation.
-
2.
Pool all samples at an equivalent DNA amount and measure the total pool DNA concentration with Fluorometric quantitation.
-
3.
Dilute each pooled sample to a final DNA concentration of 12 ng/ml with Low TE-buffer (Qubit measured). Alternatively, library quantification can be performed with the Ion Library TaqMan Quantification Kit (220–250 pM final concentration).
-
4.
Run Ion Torrent on a 318-chip (Ion PGM) or 5S Chip (Ion GeneStudio S5) for a total of 24–32 samples, according to your local Sequence Facility (see Note 30).
3.4 Illumina Protocol for IG-NGS Clonality Assessment
This two-step PCR protocol is based on a previously published protocol for marker identification for MRD [21], with some modifications for the first PCR reaction (Table 1). Furthermore, the protocol described below is optimized for sequencing on a MiniSeq (Illumina), but other equipment may be used according to the instructions of the local Sequence Facility.
3.4.1 Multiplex PCR for Amplification of IGH-FR3, IGHD, and IGK
-
1.
Prepare three different 0.2 ml PCR tubes per sample: IGH-FR3, IGHD, and IGK (see Table 1, panel “Two-step Illumina protocol for clonality detection”).
-
2.
Add 40 ng DNA (Qubit measured; see Note 14) and the other components of the reaction, according to Table 1 (panel “Two-step Illumina protocol for clonality detection”). Adjust the total PCR volume to 25 μl with MQ.
-
3.
Use a pipette to mix the prepared PCR reaction with sample thoroughly while avoiding air bubbles in the reaction mix and perform a quick spin to collect all liquid to the bottom of the PCR tube.
-
4.
Run the PCR in a thermocycler with heated lid, according to the program in Table 1 (panel “Two-step Illumina protocol for clonality detection”).
-
5.
After completion of the PCR protocol, combine tube IGH-FR3, IGHD, and IGK per sample (~75 μl total volume).
3.4.2 Cleanup of IGH-FR3, IGHD, and IGK Amplicons
-
1.
Allow the AMPure XP magnetic beads to warm to room temperature for at least 30 min before use. Ensure that the beads are homogeneous prior to use by mixing the tube by hand for 20 s (see Note 18).
-
2.
Pipette the pooled samples in a DNA LoBind plate and add 1.8 times (135 μl) volume Agencourt AMPure XP magnetic beads per sample (see Note 19).
-
3.
Use a pipette to mix the solution thoroughly (avoid air bubbles), until the beads and sample are homogeneously mixed and incubate for 5 min at room temperature.
-
4.
Place the samples for 2–5 min in a magnetic stand until the solution is clear (see Note 20).
-
5.
Carefully remove the supernatant using a 200 μl pipette (see Note 21).
-
6.
Add 150 μl freshly made 70% ethanol per sample (see Note 22).
-
7.
Move the plate in the magnetic stand approximately 4 times from left to right, and make sure the bead pellet migrates and is washed clean.
-
8.
Carefully remove the supernatant using a 200 μl pipette (see Note 21).
-
9.
Repeat steps 6–8 once.
-
10.
Carefully remove any remaining supernatant using a 10 μl pipette, and air-dry the beads for 5 min to allow complete evaporation of residual ethanol (see Note 23).
-
11.
Resuspend the samples in 25 μl Low TE-buffer.
-
12.
Use a pipette to mix the solution thoroughly (avoid air bubbles), to generate a homogeneously mixed solution, and incubate for 2 min at room temperature.
-
13.
Place the samples for 2 min in the magnetic stand until the solution is clear (see Note 20).
-
14.
Collect the purified DNA by pipetting the solution (~25 μl) into a new PCR strip (see Note 24).
3.4.3 Second PCR to Generate Barcoded Amplicons
-
1.
Dilute the purified amplicons 1:50 in Low TE-buffer.
-
2.
Use a Roche FastStart™ High Fidelity PCR kit (Sigma-Aldrich) to prepare a PCR mix for each sample according to Table 11. Make sure that a unique barcode combination is used for each sample (see Note 31).
-
3.
Perform the PCR reaction in a total reaction volume of 25 μl. Mix thoroughly and spin down. Make sure that all reagents are at the bottom of the tube and avoid air bubbles.
-
4.
Run the PCR in a thermocycler with heated lid, according to the program in Table 12.
-
5.
After completion of the second PCR, continue with a double purification as described in Subheading 3.4.4.
3.4.4 Cleanup of Barcode-Labeled Amplicons
The cleanup procedure described below is based on a double purification procedure, where the first step (0.6× volume beads) is a negative selection and the second step (0.25× volume beads) a positive selection. This double purification can be replaced by a single purification protocol, as described in Subheading 3.4.2. In that case, use 1.0× volume beads for the products from tubes IGHV-FR3 and IGK and 0.9× volume beads for the products from tube IGHD.
-
1.
Allow the AMPure XP magnetic beads to warm to room temperature for at least 30 min before use. Ensure that the beads are homogeneous prior to use by mixing the tube by hand for 20 s (see Note 18).
-
2.
Transfer the PCR reaction from each sample (25 μl) in a DNA LoBind plate (one sample per well), and add 0.6× volume (15 μl) Agencourt AMPure XP magnetic beads per sample (see Note 19).
-
3.
Use a pipette to mix the solution thoroughly (avoid air bubbles), until the beads and sample are homogeneously mixed, and incubate for 5 min at room temperature.
-
4.
Put the plate for 2–5 min in a magnetic stand, or until the solution is clear (see Note 20).
-
5.
Carefully transfer the supernatant (40 μl) into a new well (see Note 21).
-
6.
Remove the plate from the magnetic stand, and add 0.25× volume (10 μl) magnetic beads per sample (see Note 18).
-
7.
Use a pipette to mix the solution thoroughly (avoid air bubbles), until the beads and sample are homogeneously mixed, and incubate for 5 min at room temperature.
-
8.
Put the plate on the magnetic rack and incubate for 5 min.
-
9.
Remove and discard the supernatant.
-
10.
Add 200 μl freshly made 70% ethanol to each sample to wash the beads (see Note 22).
-
11.
Move plate in the magnetic stand approximately 4 times from left to right, and make sure the bead pellet migrates and is washed clean.
-
12.
Carefully remove and discard the supernatant (see Note 21).
-
13.
Repeat steps 10–12 once.
-
14.
Carefully remove any remaining supernatant using a 10 μl pipette, and air-dry the beads for 5 min to allow complete evaporation of residual ethanol (see Note 23).
-
15.
Remove the plate from the magnet and add 10 μl Low TE-buffer per sample.
-
16.
Mix thoroughly (avoid air bubbles) and incubate for 2 min at room temperature.
-
17.
Put the plate on the magnetic stand for 2 min or until the solution is clear (see Note 20).
-
18.
Collect the purified DNA by pipetting the solution (~10 μl) into a new PCR strip.
-
19.
Continue with setting up the Illumina sequencing run.
3.4.5 Illumina Sequencing Run
-
1.
Measure the DNA concentration of all samples using Fluorometric Quantitation.
-
2.
Pool all samples equimolar and measure the concentration of the total sample pool using fluorometric quantitation.
-
3.
Perform sequencing on the Illumina instrument employing corresponding reagent Kit, according to the manufacturer’s instructions and your local Sequence Facility (see Note 32).
3.5 Post-Analytical Data Analysis
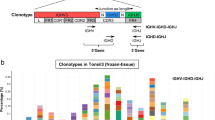
The obtained sequencing results can be analyzed with the bioinformatics tool ARResT/Interrogate (http://arrest.tools/interrogate) [18] (see Note 33). FastQ data files are uploaded and processed for analysis. Subsequently, the results can be visualized for further analysis using the “reporting” or “questions” sections. Here, the different rearrangements are referred to as clonotypes that include information about the 5′ gene, junction, and 3′ gene, as shown in Fig. 4.
Clonotype annotation. A rearrangement (complete IGH and IGK rearrangements) is referred to as a clonotype notated as an immunoglobulin nucleotide sequence with a 5′ gene (V-gene), the junction, and the 3′ gene (J-gene). The junction consists of three parts: the first and last numbers are the amount of nucleotides that is removed from the 5′- or 3′-genes, respectively. The middle number is the amount of nucleotides that is present between the 5′- and 3′-genes and includes the so-called N-nucleotides that are added by the enzyme terminal deoxynucleotidyl transferase (TdT) during the V(D)J recombination process, as well as the D-gene in case of a complete VDJ rearrangement. Incomplete, nonfunctional IGH and IGK rearrangements (IGHD-IGHJ, IGKV-KDE, Intron RSS-KDE) are “artificially” described as clonotypes in ARResT/Interrogate in a similar way as shown here for complete IGH rearrangements , using the corresponding 5′- and 3′-genes and their junctions
For basic clonality analysis, it is recommended to use the reporting function. Here, a complete overview of the clonality results is generated automatically for all IG loci, i.e., IGHV-IGHD-IGHJ (FR3), IGHD-IGHJ, and IGKV-IGKJ and a combined result for IGKV/Intron RSS-KDE, comparable with conventional clonality testing using BIOMED-2/EuroClonality assays. Select a sample, make sure the correct target is selected (i.e., choose “IG ” under cell type for B-cell clonality assessment), the filter is set on 0–100% to include all detected clonotypes, and click on “report”. The following information will be shown in the report that is generated:
-
1.
First, an overview of some quality parameters is shown. The most important is the QC status: “Pass” when the data meets all quality criteria or “Fail” when the data does not meet all quality criteria. Under “QC report” it can be found why the QC failed and which target failed; please interpret these targets with caution.
-
2.
A bar chart for each IG locus is created with the abundancy of detected clonotypes on the y-axis and the junction amino acid (aa) length on the x-axis. Note IGHD-IGHJ and all rearrangements involving KDE are not expressed and are “artificially” described as clonotypes in ARResT/Interrogate. In this way, each bar represents all clonotypes with the same junction aa length, whereas each clonotype with a unique nucleotide sequence is depicted with a specific color. Please note that only the top 50 most abundant clonotypes are colored; all other clonotypes are merged and represented by gray bars.
-
3.
A table with more detailed information about the top 15 clonotypes is generated and shown next to the bar chart. This table includes the genes and segmentation of the clonotypes, the amino acid sequence of the junction, functionality of the rearrangement (pro, productive; pop, potentially productive, for incomplete rearrangements; unp, unproductive; unk, unknown, for special rearrangements), the length of the junction in amino acids, and the number and percentage of reads with the specific clonotype and length.
-
4.
In a small-sized table, some general information (“stats for junction class set”) is shown, of which especially the total number of reads (for normalization) is important.
With the “PDF” button that is present in the reporting function, the total report can be exported as a PDF file.
More advanced analyses can be performed using the “questions” function. In addition to the standard parameters (i.e., junction aa length and clonotype ), also other ones can be chosen, like amplicon length or the 5′- or 3′-genes/primers to analyze the data in more detail. Furthermore, in contrast to the reporting section, it is possible to select 2 or more samples at the same time in the questions section, to directly compare the nucleotide sequences in either a bar chart or table. This is especially of added value when a clonal comparison has to be made for a patient with multiple tumors, for example. Using the questions function, the data can be visualized as follows:
-
1.
The “table” subsection can be used to create a table of all detected clonotypes with information about the chosen parameters. This complete table can be exported using the small download button (download full table in .xlsx format). Again, it is possible to select one or more samples for the analysis.
-
2.
Using the “bars” subsection, bar charts can be created with the parameters of interest. This can be done for a single sample or for the analysis of multiple samples simultaneously in case of a clonal comparison for example. The generated bar charts can be downloaded as image by using the button at the upper right corner next to the legend.
Within ARResT/Interrogate, all bar charts (created within both the reporting and questions function) are “interactive” meaning that by clicking on one or more colored parts of a bar, the corresponding clonotypes are selected. A so-called minitable pops up at the top of the page, with the general information about the clonotype (s), but also the most popular full nucleotide sequence of the corresponding clonotype . This information can be downloaded using the download button. Further analysis of the most popular nucleotide sequence, but also all other sequences belonging to the same clonotype , can be done within the “forensics” function. By using the green button “run tests,” this forensics section will open automatically or go manually to this section. Here, the following analyses can be performed:
-
1.
When forensics is opened, automatically the subsection “tests” is shown. By selecting “interrogate” and choosing “run the test on minitable,” more detailed information will appear for the most popular nucleotide sequence, like the segmentation of the clonotype and alignment to the germline sequences of the corresponding genes. Also additional tools (i.e., IMGT/V-QUEST and Vidjil) are available for further analysis of the selected clonotypes.
-
2.
By going to the subsection “sequences,” a table with all nucleotide sequences, including the number of reads, is shown that corresponds to the selected clonotype which can be retrieved and downloaded. Please note that when working via the “reporting” section, first “reporting panel features” needs to be selected before sequences can be retrieved.
After visualization of the results, the obtained clonality patterns of each sample, which is run under standardized conditions (input of DNA and number of samples per run), can be interpreted. It is strongly advised to include a polyclonal control sample in the run. A standardized input of an FFPE-derived polyclonal control sample under standardized run conditions should demonstrate a Gaussian curve with differently sized junctions of the gene rearrangement and a high variety of clonotypes represented by the presence of gray bars as shown in the top panel of Fig. 5, as well as the detection of the V/D/J gene families. Skewing of the curve to either short or long amplicon lengths could imply that the library preparation was not optimal and may interfere with the analysis of samples prepared within the same run. The same holds true for too few reads and/or clonotypes. Depending on the tumor load of a clonal sample, as well as the input of DNA (per PCR-library), a dominant clonotype will be present, as shown in the lower panel of Fig. 5.
Output data generated by ARResT/Interrogate with IG-NGS clonality assay. IG-NGS clonality profiles of a polyclonal (upper panel) and monoclonal (lower panel) sample are shown in bar charts generated by ARResT/Interrogate (target IGHV-IGHD-IGHJ FR3). On the y-axis, the abundancy of detected clonotypes is shown in percentage and on the x-axis, the junction length is shown in amino acids (aa). Each bar represents clonotypes with the same junction aa length, and each color indicates a unique clonotype based on their nucleotide sequence . Only the 50 most abundant clonotypes are colored, and all other, less frequent gene rearrangements are merged and represented by the gray bars
For correct interpretation of the clonality assay per sample, several steps should be followed:
-
1.
Make a technical interpretation of the obtained results per locus and rearrangement type (i.e., IGHV-IGHD-IGHJ, IGHD-IGHJ, IGKV-IGKJ, and IGKV/Intron RSS-KDE). This includes the number of obtained reads, but also the number of different clonotypes detected, the run conditions, the DNA input, and the tumor load. Based on this protocol, the data can be analyzed reliably when at least 1000 reads are available for a PCR-target as evaluated in the EuroClonality-NGS biological validation study [22]. The technical interpretation per PCR target uses the terminology: “clonal,” “polyclonal,” or “no specific product,” with the possibility to add a more detailed information, similar as described in the guidelines for conventional clonality testing [17].
-
2.
Evaluation of the technical interpretation of the individual rearrangement types (PCRs) into a molecular clonality conclusion, according to the EuroClonality guidelines for conventional clonality testing [17].
Guidelines for the technical interpretation of the obtained result per locus and rearrangement type, as well as for the molecular clonality conclusion, are under development. Furthermore, it should be stressed that the clonality results should be integrated with the clinical, morphological, and immunophenotypic data to make a final diagnosis.
4 Notes
-
1.
The design of the two-step Illumina protocol for clonality detection is based on two previously developed assays: the Ion Torrent protocol for clonality detection [6] and the two-step Illumina protocol for marker identification [21]. The first step, i.e., target amplification, is based on the Ion Torrent protocol, since the analyzed targets are identical. Therefore, the PCR conditions and primer sequences for target amplification are the same for both protocols, except the M13 sequence on the forward and reverse primers. Due to the occurrence of SHM in most B-cell lymphomas within the IGHV genes, the annealing temperature for IGHV-FR3 PCR is lower (60 °C) in the two-step Illumina clonality detection protocol compared to the MRD marker identification protocol (63 °C). To create uniformity in the PCR programs for all targets, the PCR programs are similar for all targets.
-
2.
The chosen DNA extraction method should significantly reduce protein and RNA contamination, and thus a procedure that includes column-based purification is strongly recommended. Extraction methods that isolate both DNA and RNA in parallel are not suitable. RNA present in the DNA solution negatively influences the PCR reaction resulting in an abnormal, disturbed polyclonal pattern. The DNA should be quantified to enable standardized DNA input in the PCR.
-
3.
Different primer pools need to be prepared for each of the three multiplex PCR reactions (IGHV-FR3, IGHD, and IGK ). Each primer pool should preferably contain all forward and reverse primers for that specific target. It is recommended to prepare 25× concentrated primer pools, where 1 μl primer pool can be used for each PCR reaction. Within the pool, 5 μM or 10 μM concentrations of each primer should be added, according to Tables 2, 3, and 4, which yields 0.2 μM or 0.4 μM final concentration within the reaction, respectively. For instance, if 600 μl primer pool is prepared, add the different primer volumes from 300 μM stock concentrations, i.e., 10 μl for 5 μM primer pool concentration and 20 μl for 10 μM primer pool concentration, and adjust the final volume to 600 μl with Low TE-buffer. Please note that for the Ion Torrent protocol, the primer sequences without the M13 adapter (blue or green sequences) should be used.
-
4.
To assess whether the tissue slices used for DNA extraction is representative for the disease, 4 μm haematoxylin-eosin sections just before and after these slices should be evaluated by an experienced hematopathologist. The tissue fixation protocol may affect the degradation of the extracted DNA and thus the DNA quality in terms of amplifiability. It is important that neutral-buffered formalin is used. Prolonged fixation should be avoided as this induces too much cross-linking between DNA and other biomolecules resulting in inferior DNA quality for molecular analysis. In case the sample surface of the paraffin block has been exposed to air, it is advised to discard the first 2–3 sections before cutting sections for DNA isolation to avoid contamination. It is strongly advised to assess the quality of the purified DNA from FFPE samples by a quality control procedure using a size ladder PCR [13] and gel system, TapeStation (Agilent) or Bioanalyzer (Agilent).
-
5.
A negative control sample should be included to monitor possible contaminations throughout the entire procedure. To this end, H2O can be used as non-template negative control. A polyclonal positive control sample is essential as it allows to evaluate whether the multiplex PCR reaction was successful and to identify the V and J genes in the polyclonal sample. To obtain a complete, polyclonal IG pattern, tonsil or reactive lymph node DNA is preferred because of the higher B-cell numbers compared to peripheral blood samples.
-
6.
For deparaffinization of FFPE tissue sections, Deparaffinization Solution (QIAGEN) can be used instead of xylene. In that case, replace steps 2 until 14 for the manufacturer’s protocol supplied with the deparaffinization solution, and continue the protocol with step 15.
-
7.
It is important that the tissue sample is completely lysed for optimal DNA yields. If the tissue is not yet lysed completely after overnight incubation, add additional proteinase K (15–20 μl), and incubate at 56 °C for a few hours on a thermomixer until all tissue is dissolved.
-
8.
In case one heating block is used, leave the sample(s) at room temperature after the 56 °C incubation until the temperature within the heating block has reached 90 °C or 95 °C. Also, be aware that longer incubation at 90 °C/95 °C may result in more fragmented DNA.
-
9.
The DNA sample, buffer AL, and ethanol should be mixed immediately and thoroughly by vortexing or pipetting. When processing multiple samples, buffer AL and ethanol can be premixed and added together in one step.
-
10.
If the lysate has not completely passed through the membrane after centrifugation, centrifuge again at a higher speed until the QIAamp MinElute column is empty.
-
11.
Buffers AW1 and AW2 are provided as concentrated solutions. Make sure that ethanol has been added to prepare Buffer AW1 and Buffer AW2 in a correct way before use.
-
12.
When buffer AW2, containing ethanol, is not removed completely, this will end up in the eluate and may interfere with downstream applications.
-
13.
Ensure that the elution buffer is equilibrated to room temperature, and add the elution buffer onto the center of the membrane to ensure complete elution of bound DNA. This is especially important when elution volumes <50 μl are used.
-
14.
For the subsequent PCR step (both Ion Torrent and Illumina protocol), 40 ng input DNA is standardly used per reaction as measured by Qubit, which yields optimal results. With limited DNA stock available, it is possible to go as low as 10 ng input DNA per PCR reaction [6].
-
15.
DNA extraction can be performed at a later time point. To do so, tissue sections should be placed in an empty 1.5 ml Eppendorf tube and stored at −80 °C until further processing.
-
16.
After ethanol is added and mixed, the sample can also be incubated for up to a few hours in the fridge. This could potentially increase the DNA yield obtained after the complete procedure.
-
17.
When multiple samples are prepared at the same time, a master mix can be made containing the shared components of the different reactions. This will save time and generates uniform reaction mixtures. Please note that such master mix should be prepared for the number of samples +10% or one extra sample, kept cool (not frozen), and the polymerase is added as a final component.
-
18.
Temperature can alter the behavior of magnetic beads. These are tested and optimized for use at room temperature. Also make sure that the beads are homogenized before use, so the DNA to bead ratio is correct.
-
19.
Agencourt AMPure XP magnetic beads can also be pipetted into the DNA LoBind plate first for each sample, after which the pooled samples are added to the beads.
-
20.
Make sure that the beads form a compact pellet before removing the supernatant. If not, move the plate a little bit around the magnet, and allow them to form a more distinctive compact pellet.
-
21.
Be aware that as little beads as possible are taken up. This will lower the target specific amplicon yield after purification, as these molecules are bound to the beads. In Subheading 3.4.4, step 5, the target specific amplicons are in the supernatant, while undesired molecules are bound to the magnetic beads. Pipetting too many beads along with the supernatant can disturb downstream applications. To be sure no beads are transferred together with the supernatant, it is advised to transfer a smaller sample volume (e.g. 39 μl) and adjust with the appropriate beads volume in the next step.
-
22.
It is recommended to use a freshly prepared 70% ethanol solution to wash the samples/beads, to ensure that the ethanol concentration is correct.
-
23.
It is important that all residual ethanol is evaporated, but make sure not to overdry the beads as this will lower the DNA yield after recovery.
-
24.
After each cleanup, samples can be stored at 4 °C for up to 1 week before continuing to the next step.
-
25.
This protocol uses half the amount of reagents per sample as advised with the Ion Xpress kit. This has been tested extensively and works fine for this specific assay.
-
26.
To ensure a constant room temperature, it is recommended to perform this incubation step in a thermocycler at 20 °C.
-
27.
It is very important to use the Ion P1 Adapter from the barcode kit and not the adapters that are provided in the Ion Plus Fragment Library kit (green lid). In case the incorrect adapters are used, the barcode ligation will be very inefficient and up to 85% of the generated reads will not be barcoded and are useless.
-
28.
For the preparation of samples for one Ion Torrent run containing 24–32 samples, the same number of different barcodes is required. Each kit of Ion Xpress™ Barcode Adapters contains 16 different barcodes, so at least 2 kits are required for a library of 24–32 samples.
-
29.
When collecting the supernatant containing the amplicon products, only 12.5 μl is collected into new PCR strip for this step, to ensure that the pellet is not disturbed. Furthermore, in this way the samples are ready to be used for the next step which requires 12.5 μl of pooled amplicons.
-
30.
For sequencing and sample pool preparation, always follow the protocols and instructions from the local Sequence Facility. There are different Ion Torrent sequencers with associated kits and chips (e.g., Ion PGM Template OT2 200 Kit, Ion 510TM & Ion 520TM & Ion 530TM Kit Chef, Ion Chef [or Ion OneTouch 2 System]), which may require different concentrations and volumes of the prepared sample pool for optimal results. For running a 318 chip on the Ion Chef, it is recommended to include 24–32 samples per run.
-
31.
When preparing a set of multiple samples, PCR master mixes can be used. Depending on the choices of the barcodes primers and the number of samples, one of the primers (i.e., forward or reverse) can eventually be included in the master mix. Otherwise, add each barcoded primer separately to each sample. Please note that each sample analyzed in a single sequencing run should get a unique combination of a forward and reverse barcode.
-
32.
For sequencing and sample pool preparation, always follow the protocols and instructions from the local Sequence Facility. There are different Illumina sequencers that can be used, with their associated kits and chips for sequencing (e.g., MiniSeq sequencer and MiniSeq Mid Output Kit, or MiSeq sequencer and MiSeq Reagent Kit v2). Depending on the sequencing equipment, different concentrations and volumes of the prepared sample pool may be required for optimal results. For example, sequencing on a MiniSeq instrument using a mid-output chip requires a sample pool of 4 nM in a volume of 20 μl. Also the number of samples that can be analyzed in a single sequencing run depends on the sequencing instrument and chip. For running a mid-output chip on a MiniSeq instrument, it is recommended to include 24–32 samples per run.
-
33.
ARResT/Interrogate is best viewed using Google Chrome or Firefox. The availability of the below described functions (i.e., “reporting” and “questions”) depends on the user mode. However, in each user mode, at least one of these functions is available. For specific questions regarding an ARResT/Interrogate account, please contact the ARResT team (contact@arrest.tools).
References
Melchers F (2015) Checkpoints that control B cell development. J Clin Invest 125(6):2203–2210. https://doi.org/10.1172/jci78083
Pieper K, Grimbacher B, Eibel H (2013) B-cell biology and development. J Allergy Clin Immunol 131(4):959–971. https://doi.org/10.1016/j.jaci.2013.01.046
Rajewsky K (1996) Clonal selection and learning in the antibody system. Nature 381(6585):751–758. https://doi.org/10.1038/381751a0
Kim DR, Park SJ, Oettinger MA (2000) V(D)J recombination: site-specific cleavage and repair. Mol Cells 10(4):367–374
Gellert M (2002) V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem 71:101–132. https://doi.org/10.1146/annurev.biochem.71.090501.150203
Scheijen B, Meijers RWJ, Rijntjes J, van der Klift MY, Möbs M, Steinhilber J, Reigl T, van den Brand M, Kotrová M, Ritter JM, Catherwood MA, Stamatopoulos K, Brüggemann M, Davi F, Darzentas N, Pott C, Fend F, Hummel M, Langerak AW, Groenen P (2019) Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by EuroClonality-NGS. Leukemia 33(9):2227–2240. https://doi.org/10.1038/s41375-019-0508-7
Sanchez ML, Almeida J, Gonzalez D, Gonzalez M, Garcia-Marcos MA, Balanzategui A, Lopez-Berges MC, Nomdedeu J, Vallespi T, Barbon M, Martin A, de la Fuente P, Martin-Nuñez G, Fernandez-Calvo J, Hernandez JM, San Miguel JF, Orfao A (2003) Incidence and clinicobiologic characteristics of leukemic B-cell chronic lymphoproliferative disorders with more than one B-cell clone. Blood 102(8):2994–3002. https://doi.org/10.1182/blood-2003-01-0045
Collins AM, Watson CT (2018) Immunoglobulin light chain gene rearrangements, receptor editing and the development of a self-tolerant antibody repertoire. Front Immunol 9:2249. https://doi.org/10.3389/fimmu.2018.02249
van Zelm MC, van der Burg M, de Ridder D, Barendregt BH, de Haas EF, Reinders MJ, Lankester AC, Révész T, Staal FJ, van Dongen JJ (2005) Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol 175(9):5912–5922. https://doi.org/10.4049/jimmunol.175.9.5912
Rees AR (2020) Understanding the human antibody repertoire. MAbs 12(1):1729683. https://doi.org/10.1080/19420862.2020.1729683
Neuberger MS, Milstein C (1995) Somatic hypermutation. Curr Opin Immunol 7(2):248–254. https://doi.org/10.1016/0952-7915(95)80010-7
Gazzola A, Mannu C, Rossi M, Laginestra MA, Sapienza MR, Fuligni F, Etebari M, Melle F, Sabattini E, Agostinelli C, Bacci F, Sagramoso Sacchetti CA, Pileri SA, Piccaluga PP (2014) The evolution of clonality testing in the diagnosis and monitoring of hematological malignancies. Ther Adv Hematol 5(2):35–47. https://doi.org/10.1177/2040620713519729
van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia 17(12):2257–2317. https://doi.org/10.1038/sj.leu.2403202
Boone E, Heezen KC, Groenen P, Langerak AW (2019) PCR GeneScan and heteroduplex analysis of rearranged immunoglobulin or T-cell receptor genes for clonality diagnostics in suspect lymphoproliferations. Methods Mol Biol 1956:77–103. https://doi.org/10.1007/978-1-4939-9151-8_4
Evans PA, Pott C, Groenen PJ, Salles G, Davi F, Berger F, Garcia JF, van Krieken JH, Pals S, Kluin P, Schuuring E, Spaargaren M, Boone E, Gonzalez D, Martinez B, Villuendas R, Gameiro P, Diss TC, Mills K, Morgan GJ, Carter GI, Milner BJ, Pearson D, Hummel M, Jung W, Ott M, Canioni D, Beldjord K, Bastard C, Delfau-Larue MH, van Dongen JJ, Molina TJ, Cabecadas J (2007) Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 concerted action BHM4-CT98-3936. Leukemia 21(2):207–214. https://doi.org/10.1038/sj.leu.2404479
van Krieken JH, Langerak AW, Macintyre EA, Kneba M, Hodges E, Sanz RG, Morgan GJ, Parreira A, Molina TJ, Cabeçadas J, Gaulard P, Jasani B, Garcia JF, Ott M, Hannsmann ML, Berger F, Hummel M, Davi F, Brüggemann M, Lavender FL, Schuuring E, Evans PA, White H, Salles G, Groenen PJ, Gameiro P, Pott C, Dongen JJ (2007) Improved reliability of lymphoma diagnostics via PCR-based clonality testing: report of the BIOMED-2 concerted action BHM4-CT98-3936. Leukemia 21(2):201–206. https://doi.org/10.1038/sj.leu.2404467
Langerak AW, Groenen PJ, Bruggemann M, Beldjord K, Bellan C, Bonello L, Boone E, Carter GI, Catherwood M, Davi F, Delfau-Larue MH, Diss T, Evans PA, Gameiro P, Garcia Sanz R, Gonzalez D, Grand D, Hakansson A, Hummel M, Liu H, Lombardia L, Macintyre EA, Milner BJ, Montes-Moreno S, Schuuring E, Spaargaren M, Hodges E, van Dongen JJ (2012) EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 26(10):2159–2171. https://doi.org/10.1038/leu.2012.246
Bystry V, Reigl T, Krejci A, Demko M, Hanakova B, Grioni A, Knecht H, Schlitt M, Dreger P, Sellner L, Herrmann D, Pingeon M, Boudjoghra M, Rijntjes J, Pott C, Langerak AW, Groenen P, Davi F, Bruggemann M, Darzentas N (2017) ARResT/Interrogate: an interactive immunoprofiler for IG/TR NGS data. Bioinformatics 33(3):435–437. https://doi.org/10.1093/bioinformatics/btw634
Merriman B, Rothberg JM (2012) Progress in ion torrent semiconductor chip based sequencing. Electrophoresis 33(23):3397–3417. https://doi.org/10.1002/elps.201200424
Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, Boutell JM, Bryant J, Carter RJ, Keira Cheetham R, Cox AJ, Ellis DJ, Flatbush MR, Gormley NA, Humphray SJ, Irving LJ, Karbelashvili MS, Kirk SM, Li H, Liu X, Maisinger KS, Murray LJ, Obradovic B, Ost T, Parkinson ML, Pratt MR, Rasolonjatovo IM, Reed MT, Rigatti R, Rodighiero C, Ross MT, Sabot A, Sankar SV, Scally A, Schroth GP, Smith ME, Smith VP, Spiridou A, Torrance PE, Tzonev SS, Vermaas EH, Walter K, Wu X, Zhang L, Alam MD, Anastasi C, Aniebo IC, Bailey DM, Bancarz IR, Banerjee S, Barbour SG, Baybayan PA, Benoit VA, Benson KF, Bevis C, Black PJ, Boodhun A, Brennan JS, Bridgham JA, Brown RC, Brown AA, Buermann DH, Bundu AA, Burrows JC, Carter NP, Castillo N, Chiara ECM, Chang S, Neil Cooley R, Crake NR, Dada OO, Diakoumakos KD, Dominguez-Fernandez B, Earnshaw DJ, Egbujor UC, Elmore DW, Etchin SS, Ewan MR, Fedurco M, Fraser LJ, Fuentes Fajardo KV, Scott Furey W, George D, Gietzen KJ, Goddard CP, Golda GS, Granieri PA, Green DE, Gustafson DL, Hansen NF, Harnish K, Haudenschild CD, Heyer NI, Hims MM, Ho JT, Horgan AM, Hoschler K, Hurwitz S, Ivanov DV, Johnson MQ, James T, Huw Jones TA, Kang GD, Kerelska TH, Kersey AD, Khrebtukova I, Kindwall AP, Kingsbury Z, Kokko-Gonzales PI, Kumar A, Laurent MA, Lawley CT, Lee SE, Lee X, Liao AK, Loch JA, Lok M, Luo S, Mammen RM, Martin JW, McCauley PG, McNitt P, Mehta P, Moon KW, Mullens JW, Newington T, Ning Z, Ling Ng B, Novo SM, O'Neill MJ, Osborne MA, Osnowski A, Ostadan O, Paraschos LL, Pickering L, Pike AC, Pike AC, Chris Pinkard D, Pliskin DP, Podhasky J, Quijano VJ, Raczy C, Rae VH, Rawlings SR, Chiva Rodriguez A, Roe PM, Rogers J, Rogert Bacigalupo MC, Romanov N, Romieu A, Roth RK, Rourke NJ, Ruediger ST, Rusman E, Sanches-Kuiper RM, Schenker MR, Seoane JM, Shaw RJ, Shiver MK, Short SW, Sizto NL, Sluis JP, Smith MA, Ernest Sohna Sohna J, Spence EJ, Stevens K, Sutton N, Szajkowski L, Tregidgo CL, Turcatti G, Vandevondele S, Verhovsky Y, Virk SM, Wakelin S, Walcott GC, Wang J, Worsley GJ, Yan J, Yau L, Zuerlein M, Rogers J, Mullikin JC, Hurles ME, McCooke NJ, West JS, Oaks FL, Lundberg PL, Klenerman D, Durbin R, Smith AJ (2008) Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456(7218):53–59. https://doi.org/10.1038/nature07517
Bruggemann M, Kotrova M, Knecht H, Bartram J, Boudjogrha M, Bystry V, Fazio G, Fronkova E, Giraud M, Grioni A, Hancock J, Herrmann D, Jimenez C, Krejci A, Moppett J, Reigl T, Salson M, Scheijen B, Schwarz M, Songia S, Svaton M, van Dongen JJM, Villarese P, Wakeman S, Wright G, Cazzaniga G, Davi F, Garcia-Sanz R, Gonzalez D, Groenen P, Hummel M, Macintyre EA, Stamatopoulos K, Pott C, Trka J, Darzentas N, Langerak AW (2019) Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia 33(9):2241–2253. https://doi.org/10.1038/s41375-019-0496-7
van den Brand M, Rijntjes J, Mobs M, Steinhilber J, van der Klift MY, Heezen KC, Kroeze LI, Reigl T, Porc J, Darzentas N, Luijks J, Scheijen B, Davi F, ElDaly H, Liu H, Anagnostopoulos I, Hummel M, Fend F, Langerak AW, Groenen P, EuroClonality NGSWG (2021) Next-generation sequencing-based clonality assessment of Ig gene rearrangements: A multicenter validation study by euroClonality-NGS. J Mol Diagn 23(9):1105–1115
Acknowledgments
The development of the NGS-based protocols for clonality detection was executed by laboratories within the EuroClonality-NGS Working Group, part of the EuroClonality consortium. A special thanks to Jos Rijntjes and Jeroen Luijks (Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands) for technical assistance during the development of the two-step Illumina protocol for clonality detection. This project was funded by EuroClonality and the Dutch Cancer Society (KWF-11137). Figures are created with BioRender.com.
Author information
Authors and Affiliations
Consortia
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
van Bladel, D.A.G., van der Last-Kempkes, J.L.M., Scheijen, B., Groenen, P.J.T.A., on behalf of the EuroClonality Consortium. (2022). Next-Generation Sequencing-Based Clonality Detection of Immunoglobulin Gene Rearrangements in B-Cell Lymphoma. In: Langerak, A.W. (eds) Immunogenetics. Methods in Molecular Biology, vol 2453. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2115-8_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2115-8_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2114-1
Online ISBN: 978-1-0716-2115-8
eBook Packages: Springer Protocols