Abstract
Renal MRI holds incredible promise for making a quantum leap in improving diagnosis and care of patients with a multitude of diseases, by moving beyond the limitations and restrictions of current routine clinical practice. Clinical and preclinical renal MRI is advancing with ever increasing rapidity, and yet, aside from a few examples of renal MRI in routine use, it is still not good enough. Several roadblocks are still delaying the pace of progress, particularly inefficient education of renal MR researchers, and lack of harmonization of approaches that limits the sharing of results among multiple research groups.
Here we aim to address these limitations for preclinical renal MRI (predominantly in small animals), by providing a comprehensive collection of more than 40 publications that will serve as a foundational resource for preclinical renal MRI studies. This includes chapters describing the fundamental principles underlying a variety of renal MRI methods, step-by-step protocols for executing renal MRI studies, and detailed guides for data analysis. This collection will serve as a crucial part of a roadmap toward conducting renal MRI studies in a robust and reproducible way, that will promote the standardization and sharing of data.
This chapter is based upon work from the COST Action PARENCHIMA, a community-driven network funded by the European Cooperation in Science and Technology (COST) program of the European Union, which aims to improve the reproducibility and standardization of renal MRI biomarkers.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- Magnetic resonance imaging (MRI)
- Kidney
- Animals
- Acute kidney injury
- Chronic kidney disease
- Training
- Standardization
1 Who Is Pulling the Brakes in Renal MRI?
1.1 Renal MRI in Clinical Practice—Fantasy, Dream, or Reality?
Magnetic resonance imaging (MRI) of the kidney is not brain surgery—it’s better! It can save lives without invasive surgery, noninvasively. This statement—a play on Donald W McRobbie’s “MRI is not rocket science, it’s better” in MRI From Picture to Proton—is one that Dr. Susan Back, radiologist and director of Pediatric Genitourinary Imaging at the Children’s Hospital of Philadelphia, would sign on to without hesitation. It’s Thursday afternoon and she is just running an MRI scan on a 4-year-old boy with a left kidney urinary tract dilation, which was gradually increasing on ultrasound. This is the last sequence in the MRI protocol: a contrast-enhanced dynamic 3D T1-weighted GRE sequence with a temporal resolution of ~8 s, used for quantitative functional urography [1]. Before, an anatomic T2-weighted MR urogram [1] was performed to identify possible anatomic causes of obstruction (Fig. 1), which are difficult to find with ultrasound. These renal MRI data play a key role in the diagnosis and treatment decisions. The configuration of the kidney on the MRI is concerning for an ureteropelvic junction obstruction because there is an abrupt transition from the renal pelvis to the proximal ureter. However, having this anatomic image and the functional information generated using dynamic imaging shows good excretion and proves that the kidney is not obstructed. For the decision on whether to operate, the urologist takes a look at the quantitative functional information. Offline analysis of the dynamic data using the Parametric MRI software package (www.parametricmri.com) [2] provided a wide range of quantitative parameters for assessment of renal function, including renal transit time, calyceal transit time, volumetric differential renal function (vDRF), and Patlak differential renal function (pDRF). The other good news is that the left kidney is functioning similarly to the normal right kidney (vDRF: 48%/52%, pDRF: 49%/51%) so the urologist decides not to operate and the child would be put under observation.
Renal MRI used for diagnosis and treatment planning of a 4-year-old boy with a left kidney urinary tract dilation. The anatomic portion of the MR urogram study (left: postprocessed image created by superimposing the vascular/parenchymal enhancement phase with the renal excretion phase; right: 3D rendering) depicted the left urinary tract dilation with an abrupt transition in caliper between the dilated renal pelvis and the proximal ureter
While this scene of renal MRI in clinical routine use vividly illustrates the dream and ambition of many renal MRI researchers—clinicians (nephrologists, urologists, radiologists, surgeons, etc.), clinical scientists, MRI scientists, and basic scientists alike—it may, to the more realistic ones, be perhaps no more than a wild phantasy. However, for the vast majority of kidney patients worldwide, reality couldn’t be more different: diagnosis and treatment decisions are predominantly based on plasma and urine parameters, that are known to be insensitive and unspecific. Information on increased serum creatinine is literally too little too late. Currently, the estimated glomerular filtration rate (eGFR; commonly calculated from serum creatinine including variables for age, gender, race) is “the best overall index of kidney function” [3], but it “is an unreliable tool to assess renal function in health and disease, as well as in clinical practice and research” [4]. It is like trying to study the complex bio system of the vast Amazon river basin solely by taking water samples at the mouth of the river.
1.2 Renal MRI, Where Are You?
Renal MRI undoubtedly holds great potential to improve diagnosis and care for millions of patients. The scientific literature reveals hundreds of renal MRI studies, both in patients and animals, aiming to demonstrate its clinical value, and to detail and validate the observed changes in functional and structural parameters. On reading the introductions of these studies we are typically being reminded of the millions of patients suffering from renal disease—acute kidney injuries (AKI ) and chronic kidney diseases (CKD )—as well as the steadily growing number of diabetes patients of which many are inevitably en route to diabetic nephropathy. This is usually followed by highlighting the urgent need for more sensitive and specific bio markers, with renal MRI being a prime candidate. Yet renal MRI is still virtually absent from the radar screen of the nephrologist, and patients like those at the Children’s Hospital of Philadelphia are a rare exception. What is going wrong?
1.3 Function, Function, Function
If the three most important characteristics that determine the value of a house are often considered to be “location, location, location,” then the three most important characteristics of the kidney are “function, function, function”—rather than its structure/morphology. Morphology, however, is what conventional clinical MRI is all about in the vast majority of cases. Radiologists are trained to detect and identify the subtle deviations from “normal” morphology on grayscale images with different T1 or T2(T2*) weighting. This leads to three challenges for renal MRI: (1) assessing functional images, for example of perfusion or blood oxygenation, requires new training and learning of what “normality” means; (2) almost all renal MRI techniques provide “exotic” pseudocolored parametric maps rather than conventional weighted images; (3) interpreting changes in these MR parameters in the context of the complex renal physiology is anything but trivial—in other words: we don’t really know what they mean.
Particularly for the last point, preclinical research is crucial because it enables researchers to obtain from the same organ MRI data together with physiological parameters from invasive probes, as well as histological data. Obvious examples are the comparison of T1 and ADC with the degree of fibrosis from histology [5], ASL perfusion with invasively measured renal blood flow and local flux, or T2* with invasively measured tissue oxygenation [6]. Moreover, in preclinical studies the application of (ir)reversible experimental interventions permits studying the complex relationships between MRI parameters and quantitative physiological parameters in the context of kidney-specific control of hemodynamics and oxygenation (see the chapter by Cantow K et al. “Reversible (Patho) Physiologically Relevant Test Interventions: Rationale and Examples” ). Clearly, a lot more work needs to be done to establish and translate renal MRI into clinical practice, and preclinical research is an essential part of this process. Shedding some light on where we are with regard to research activity in MRI of the kidney may help us drive forward the development of renal MRI.
1.4 Research Activity in Renal MRI
Renal and cardiac MRI both started as niche applications, with their own unique challenges for clinical translation, ranging from acquisition to analysis and interpretation. Cardiac MRI has already become an established clinical tool, which is supported and driven by a dedicated international society (Society for Cardiovascular Magnetic Resonance (SCMR), scmr.org) and guided by >10 published consensus/position statements (scmr.org/page/guidelines). Renal MRI, on the other hand, is struggling to get off the ground.
Conceivably, this divergence is partially due to the usefulness of morphological MRI, which is rather different for both applications. Unlike cardiac MRI, with its workhorse - cardiac function assessment - being based on (cinematic) morphological images, renal MRI depends on multi-parametric structural and functional information, derived from T1, T2, BOLD, DWI, ASL, etc. A second reason might be the availability of treatment and the nature of the diseases. Due to the still rather limited treatment options for CKD , performing complicated and expensive MRI exams still seems less critical than in cardiac disease where there are more management options. It is expected that the emergence of new CKD treatments will stimulate the interest in renal MRI.
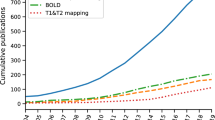
It is interesting to consider exactly how much clinical and preclinical research activity in cardiac and renal MRI there has been, and how it has grown over the years. Using publications listed in PubMed as an indicator, we performed tailored searches with PubMed’s Medical Subject Headings (MeSH) to obtain lists of literature on renal/cardiac MRI in humans/animals (Fig. 2, upper panels). Additionally we used PubMed’s MeSH Major Topic to restrict the searches to papers that focus on renal/cardiac MRI while excluding articles that only mention these terms but have another focus (Fig. 2, lower panels) (see Note 1).
The number of publications in both fields has grown considerably over the years, but at very different rates. Around 1000 cardiac MRI papers per year were published during 2014–2018; the equivalent number for renal MRI papers was only around 200. After restricting the search to papers with a main focus on MRI of the kidney/heart the number of papers per year were 400 and 70, respectively. In other words, a lot more research activity in renal MRI is needed. From the number of publications one can deduce that there is considerably more clinical research than preclinical research in renal MRI. Conceivably, this is partially due to the more limited availability of preclinical MRI systems. However, preclinical renal MRI has recently seen a rapid increase to 37% of all renal MRI publications. This highlights the importance of animal research in renal MRI, considering that only 5% of cardiac MRI papers are preclinical (as of 2018).
The renal MRI research community is still rather small. While a recent SCMR meeting attracted more than 1900 attendees [7], international meetings on renal MRI have had approximately 150–200 attendees [8, 9]. Around 200 experts in renal MRI from 30 countries are part of PARENCHIMA (renalmri.org), a community-driven Action in the COST program of the European Union, with the aim to improve the reproducibility and standardization of renal MRI biomarkers. In fact, the number of research groups active in preclinical renal MRI is only 1/5 of those active in clinical renal MRI (see Note 2). One important conclusion from this is that accelerating the development of renal MRI will require more researchers and institutions to enter the field. We may ask ourselves, “What hurdles are slowing down progress and impeding clinical translation?”
1.5 The Usual Suspects
For a novel MRI technique, the road to routine clinical use is a stony one, involving issues such as reimbursement, available time for MRI, evidence needed that the new method is superior to existing techniques, and availability of hardware/software and trained staff. However, most renal MRI techniques are still at an early phase of development. Here, learning how to correctly interpret the MRI parameters (T1, T2*, ADC , etc.) and establishing normal ranges for these MRI parameters are only two of the items on the to-do list.
When searching for the culprits for the slow development of renal MRI, we also encounter the usual suspects: lack of training and standardization. This will sound familiar to most academic researchers. The workforce of academic research consists primarily of PhD students, who typically start their MRI research lacking the most relevant knowledge, and thus spend half or even more of their 3- to 4-year project learning the necessary theory, skills, hands-on experience and the many “secret tricks” that are essential for performing successful studies but are not found in the usual literature. This is problem #1: inefficient learning.
When designing a study, researchers encounter a further difficulty. Puzzled by the great variety of protocols (and diversity of equipment) in the literature, and often lacking an explanation for the choice of parameters, they are forced to design their study based on their own rationales and gut feelings. This is problem #2: lack of standardization. This not only hampers new researchers in setting up their studies but, even more devastatingly, has a detrimental effect on the comparability and reproducibility of research. This is a major obstacle to fast and efficient research and development.
When researchers finally publish their results, they do so in the usual format of a scientific paper, which focuses on a concise description of the problem, proposed solution, main findings, and a discussion of the meaning and limitations of the study. Important details about the practicalities of actually conducting the experiments are usually omitted. When individuals leave the lab, most of their crucial experience and expertise is lost. The cycle starts again with the next student.
2 How Can Training and Standardization Be Improved?
2.1 A Roadmap for Improved Training and Standardization of Renal MRI
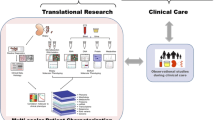
Let’s recap: the box of diagnostic tools available to the nephrologists is still rather poorly equipped (mostly insensitive and nonspecific plasma/urine markers), and renal MRI has the potential to be a game changer for the treatment of AKI and CKD . To address the numerous challenges of clinical translation of renal MRI, much more research activity is needed, and the community needs to grow. Among the factors preventing rapid progress are inefficient learning and lack of standardization. To overcome these roadblocks we suggest a roadmap for improving the training and standardization of preclinical and clinical renal MRI (Fig. 3). This combines protocol collections in the Springer Protocols book format with consensus-based technical recommendation papers based on the Delphi method.
Suggested roadmap for improving the training and standardization of preclinical and clinical renal MRI. It combines Springer Protocols books (excellent for training but also provides working protocols that help to improve comparability/standardization of future studies) with consensus-based technical recommendation papers (established tool to move toward standardization of studies). clinical = human MRI, (pre)clinical = human and animal MRI, preclinical = animal MRI
Springer Protocols is “the world’s largest collection of protocols of biomedical and life sciences,” currently with over 58,000 step-by-step experimental protocols in more than 2000 books. The protocols are published individually as electronic publications in the PubMed cited journal Methods in Molecular Biology as well as in the form of printed books, with each book representing a collection of protocols for a specific topic. “Each protocol is provided in readily-reproducible step-by-step fashion, opening with an introductory overview, a list of the materials and reagents needed to complete the experiment, and followed by a detailed procedure that is supported with a helpful notes section offering tips and tricks of the trade as well as troubleshooting advice.” [10]. This format makes Springer Protocols an excellent tool for training, and by providing working protocols, they also help to improve the reproducibility, comparability, and standardization of studies. Researchers are less likely to perform studies with “arbitrary” protocols and parameters when tested and proven protocols are readily available.
An established step toward standardization are consensus-based technical recommendation papers published as articles in technical journals. The process of developing expert consensus on technical aspects is a challenge. Here the Delphi method [11] can help to generate consensus statements: anonymous surveys that ensure all opinions are heard, free from peer pressure, are followed by rounds of face-to-face discussions. This iterative method permits “reaching reliable consensus in practice guidelines on health-care-related issues and on topics where there is little or no definitive evidence and where opinion is important” [12].
Because step-by-step protocols and technical recommendations address different ends of the training-standardization spectrum (Fig. 3), both perfectly complement each other. When deciding which step to take first, one needs to take into account the specific situation, that is, the size of the research community, the amount of hands-on experience available, the range of different equipment in use, the range of different subjects/objects being investigated, and so on. In these aspects clinical and preclinical renal MRI differ significantly. The number of research groups active in clinical renal MRI is five times the number of those active in preclinical renal MRI (see Subheading 1.4). In the clinical setting the range of setups (RF coils, field strengths) and subjects is much smaller than in preclinical settings. Even though we highlighted pediatric renal MRI in the introduction, the vast majority of renal MRI studies are performed on adult subjects at 1.5 or 3.0T. In preclinical renal MRI predominantly two species of very different size—mice and rats—are investigated at fields strengths of 3.0T, 4.7T, 7.0T, 9.4T, 11.7T, and 16.4T, with a wide range of RF coils ranging from human wrist coils to RX surface array + TX volume resonator combinations tailored for mouse or rat cardiac MRI, to cryogenically cooled TX/RX surface coils. These variations in setup influence numerous factors, including the achievable signal-to-noise ratio, the spatial resolution, and the relaxation times (T1, T2, T2*), thus leading to substantially different MRI protocols. Both the great technical variety and the small size of the preclinical renal MRI community make reaching consensus-based technical recommendations particularly challenging, because many different sets of recommendations would be needed to address all commonly used study setups. Therefore, while consensus-based technical recommendations may be the natural first step for clinical renal MRI, for preclinical MRI we decided to focus instead on creating a comprehensive Springer Protocols collection to improve training and comparability of studies.
Both, consensus-based recommendations papers and protocols collections, can only be fully effective if they are made openly accessible. Therefore, we are very glad that it was possible to make these publications open-access thanks to support from the COST Action PARENCHIMA (renalmri.org).
2.2 Driving on the Road to more Efficient Renal MRI Research: Are We There Yet?
The answer is, of course, “not yet,” as there is still some distance left to drive. But a giant leap has been made already, thanks to the COST Action PARENCHIMA (renalmri.org), which unites more than 200 experts in renal MRI from 30 countries, and the time, effort, and energy invested by more than 100 authors. After recent clinical position papers (https://academic.oup.com/ndt/issue/33/suppl_2) confirmed the demand, two projects have been implemented: The consensus-based technical recommendations for clinical translation of renal MRI (described in [12]) and the Springer Protocols book on preclinical renal MRI (described in Subheading 3).
Four papers present the results of applying the Delphi method to clinical renal MRI: arterial spin labeling (ASL) perfusion, blood oxygenation level dependent (BOLD ) MRI, diffusion-weighed imaging (DWI ), and mapping of renal T1/T2 [13,14,15,16]. This process generated over 160 consensus statements but also flagged topics where experts were currently unable to agree on a recommendation. These first ever technical recommendations for renal MRI should spark research into their appropriateness, with the aim to either prove or disprove specific recommendations. The findings of these future studies should then be fed back into updated and revised versions of the technical recommendations papers (Fig. 3). We hope that this approach will gradually lead to an alignment of the methods for measuring renal MRI biomarkers. It goes without saying that also the spectrum of methods covered must be extended in the future.
In the roadmap (Fig. 3), we propose to supplement these fresh off the press, already existing publications with the respective other parts, so that there will be step-by-step protocols as well as consensus-based technical recommendations available for both clinical and preclinical renal MRI. Here, the Springer Protocols for clinical MRI of the kidney are a placeholder for any type of protocol style journal, that is, Methods in Molecular Biology (Springer Protocols), Nature Protocols, Protocol Exchange, Journal of Visualized Experiments (JoVE), and so on. In contrast to the Springer Protocols, the latter permits independent publications on specific methods, which could be advantageous for clinical renal MRI, as it would not be necessary to publish protocols for many methods simultaneously. Needless to say, regularly updating all protocols and recommendations will be a challenging but, nevertheless, very important part of advancing the progress of renal MRI.
3 The Springer Protocols Book on Preclinical Renal MRI
Since Douglas Adams’s The Hitchhiker’s Guide to the Galaxy was first published in 1979, the number “42” has been claimed to be the answer to the question of “life, the universe, everything.” After more than 2 years of hard work by 90 authors, the answer to the question, “How can we improve the training and standardization of preclinical renal MRI?” resulted in the 42 (other) chapters of this Springer Protocols book Pohlmann A, Niendorf T (eds) (2020) “Preclinical MRI of the Kidney—Methods and Protocols,” Springer, New York. In the following subsections we describe the tailored concept of the book and the topics covered by the chapters.
3.1 Concept and Special Features of the Book
When we started to think about the structure and content of this book on preclinical renal MRI (with main focus on small animals), it immediately became clear that a simple collection of independent chapters would not suffice, for a multitude of reasons. First, there are many important aspects involved in renal MRI that are relevant to any MRI method: it does not make sense that for every individual method the protocol would include considerations and instructions regarding the MRI hardware, animal preparation, physiological monitoring, or image slice planning and shimming. Second, due to the complexity of MRI it is very challenging to include all the information about a method in a single chapter. If the book was to become a one-stop-shop for learning renal MRI in small animals, a concept was needed. In the following we describe its concept and special features.
3.1.1 Provide all the Necessary Information for each Method
With a few exceptions, we included three chapters for each renal MRI method:
-
1.
There is one chapter describing the basic concepts of the method. We believe a sound understanding of the measurement concept is essential when planning, performing, analyzing, and interpreting a study. This is complemented by a brief overview of the preclinical renal applications, in order to illustrate for what kind of questions and applications each technique is useful for.
-
2.
A step-by-step experimental protocol, which we know from the many existing Springer Protocols books. These protocols are the core of the book. For less complex methods they include also instructions for data analysis.
-
3.
Detailed step-by-step protocols for data analysis.
3.1.2 Generalize Protocols by Peer Review
Although we trusted that each author had extensive experience and described the protocols correctly and in an understandable manner, we endeavored to make sure these were free from lab specific techniques, assumptions and limitations, which could be due, for example, to the (un)availability of equipment or traditions. Because these protocols should also serve to move toward the harmonization of studies, we felt it was important to ensure that the protocols were as universal as possible and would work in many labs and settings. For this reason we implemented a coauthor peer review process, that is, every chapter had to have one or several external coauthors from other laboratories. The roles of these coauthors are as follows:
-
1.
To check whether the protocol would be applicable in other settings,
-
2.
To check whether the steps and parameters are reasonable and their rationale clear and correct,
-
3.
To add example protocols for additional lab settings.
3.1.3 Avoid Magic Numbers
Magic numbers—that is, numbers which are not explained and whose choice appears to be arbitrary—are not only a bad idea for software program codes but also for lab protocols. How often have we all read scientific publications and just could not figure out why the authors chose that particular set of measurement parameters! Was there a clever rationale behind their choice that sadly we didn’t know, a lab tradition which might be reasonable but perhaps not applicable to our setting, or just a gut feeling of a student that didn’t know how to make sense of all the different examples in the literature?
In any case, we wanted to reduce the magic numbers in our protocols to a minimum, so all authors were instructed to explain the rationales for their parameter choices in generic terms. If possible, advice for adapting them to other settings (other species, other field strengths) was to be given in the Notes section. Of course, example parameter sets are very valuable because they may allow readers to start straight away with a running protocol. They are also important in terms of harmonization, as mentioned. Therefore, examples of parameter sets for specific settings were requested, but separately in the Notes section, for example parameter sets for mice in a 7T MRI system and rats in a 9.4T system. For some techniques parameter sets for rats in a clinical 3T MRI system were also included.
3.1.4 Describe the Pragmatic Way for Data Analyses
It was important to us to dedicate separate chapters to the analyses because data analysis is an essential part of each study, but it is very rarely described in adequate detail. Statements like “T2* was calculated by pixel-wise exponential fitting to the data using an in-house developed software written in MATLAB®” don’t really help anyone who wants to learn how to analyze the data correctly. We are sure many of us have used similar statements in previous journal publications, but the fault is not always entirely ours: often there is simply no space (word limits!) and time to give a detailed description. Here, we wanted to make sure there was space (by having an entire chapter solely for the analysis) and time (by rewarding the effort and time spent in the detailed description with a first authorship).
It was key to present the most pragmatic approach to data analysis. What is the point in describing how to do your own code if there is established software available? So if analysis software existed, authors should describe how to get it and how to use it. Otherwise, the step-by-step instructions should focus on how to write a software program. Including pseudo code was encouraged, as well as providing downloadable code examples via GitHub or similar.
Finally, we asked to provide the readers with ideas on how to validate that their analyses give correct results. This could include for example a table of reference values or using synthetic test data.
3.1.5 Prevent Tunnel Vision—A Successful Study Needs More Than an MRI Protocol
Indeed, performing the actual MRI study is only part of the story. One must not forget that physiological MRI also requires consideration of many factors that are less important in anatomical imaging. Considerations about physiological monitoring, choosing the right animal model, measuring at the right time of the day, and using the most suitable anesthesia all have important implications.
For this reason, the first part of the book was dedicated to topics like animal models, preparation, monitoring and physiological interventions. We also questioned the need to always perform in vivo experiments on animals for training, development, and testing. Hence, one chapter provides a step-by-step protocol for the preparation of ex vivo rodent phantoms.
3.1.6 Embrace Competition—There Are Other Great Techniques Besides MRI
There are numerous questions for which it makes sense to go multimodal. Not only ultrasound and photoacoustic imaging but also invasive probes that provide quantitative physiological measurements are extremely valuable complements. Therefore, this book includes more than just MRI.
3.1.7 Make Access to This Information Free of Charge
Thanks to support from the COST Action PARENCHIMA (renalmri.org) it was possible to make all chapters open access!
3.2 Content of the Book
An overview of topics covered by the Springer Protocols book on preclinical renal MRI is shown in Fig. 4. Part II contains four chapters about animal models, preparation, monitoring, physiological interventions, and rodent phantoms. In Part III there are 13 chapters describing the basic concepts of the techniques, followed by Part IV with 14 step-by-step protocols for conducting experiments. Finally, Part V contains ten chapters that address data analysis; this includes the subsegmentation of the kidney into morphology-based regions of interest or concentric objects, as well as image denoising using nonlocal means (NLM) filtering.
Overview of topics covered by the Springer Protocols book on preclinical renal MRI. There are 4 chapters about animal models, preparation, monitoring, physiological interventions, and rodent phantoms; 13 chapters describing the basic concepts of the techniques; 14 chapters with step-by-step protocols for experiments, and 11 data analysis protocols
More detailed information on the book structure and chapters is provided below by listing all chapters in the format [chapter number] [chapter title] [author list]. Each chapter is PubMed-listed as an entry in Methods Mol Biol.
3.2.1 Part I—Introduction
-
1.
Recommendations for Preclinical Renal MRI: A Comprehensive Open-Access Protocol Collection to Improve Training, Reproducibility, and Comparability of Studies (Andreas Pohlmann, Susan J. Back, Andrea Fekete, Iris Friedli, Stefanie Hectors, Neil Peter Jerome, Min-Chi Ku, Dario Livio Longo, Martin Meier, Jason M. Millward, João S. Periquito, Erdmann Seeliger, Suraj D. Serai, Sonia Waiczies, Steven Sourbron, Christoffer Laustsen, and Thoralf Niendorf).
3.2.2 Part II—Animal Models, Preparation, Monitoring, and Physiological Interventions
-
1.
Animal Models of Renal Pathophysiology and Disease (Adam Hosszu, Tamas Kaucsar, Erdmann Seeliger, and Andrea Fekete).
-
2.
Preparation and Monitoring of Small Animals in Renal MRI (Tamas Kaucsar, Adam Hosszu, Erdmann Seeliger, Henning M Reimann, and Andrea Fekete).
-
3.
Reversible (Patho)physiologically Relevant Test Interventions : Rationale and Examples (Kathleen Cantow, Mechthild Ladwig-Wiegard, Bert Flemming, Andrea Fekete, Adam Hosszu, and Erdmann Seeliger).
-
4.
Preparation of Ex Vivo Rodent Phantoms for Developing, Testing, and Training MR Imaging of the Kidney and Other Organs (Jason M. Millward, Joao Periquito, Paula Ramos Delgado, Christian Prinz, Thoralf Niendorf, and Sonia Waiczies).
3.2.3 Part III—Basic Concepts of Measurement Techniques
-
5.
Quantitative Assessment of Renal Perfusion and Oxygenation by Invasive Probes : Basic Concepts (Kathleen Cantow, Roger G. Evans, Dirk Grosenick, Thomas Gladytz, Thoralf Niendorf, Bert Flemming, and Erdmann Seeliger).
-
6.
Ultrasound and Photoacoustic Imaging of the Kidney: Basic Concepts and Protocols (Sandra Meyer and Dieter Fuchs Martin Meier).
-
7.
Hardware Considerations for Preclinical Magnetic Resonance of the Kidney (Paula Ramos Delgado, Ekkehard Küstermann, André Kühne, Thoralf Niendorf, Andreas Pohlmann, and Martin Meier).
-
8.
MRI Mapping of Renal T1: Basic Concepts (Stefanie Hectors, Sabrina Doblas, Philippe Garteiser, Gwenaël Pagé, Bernard E. Van Beers, John C Waterton, and Octavia Bane).
-
9.
MRI Mapping of the Blood Oxygenation Sensitive Parameter T2* in the Kidney: Basic Concepts (Lu-Ping Li, Bradley Hack, Erdmann Seeliger, and Pottumarthi V. Prasad).
-
10.
Renal Diffusion Weighted Imaging (DWI ) for Apparent Diffusion Coefficient (ADC) , Intravoxel Incoherent Motion (IVIM ), and Diffusion Tensor Imaging (DTI ): Basic Concepts (Neil Peter Jerome, Anna Caroli, and Alexandra Ljimani).
-
11.
Dynamic Contrast Enhancement (DCE ) MRI–Derived Renal Perfusion and Filtration : Basic Concepts (Michael Pedersen, Pietro Irrera, Walter Dastrù, Frank G Zöllner, Kevin M Bennett, Scott C Beeman, G Larry Bretthorst, Joel R Garbow, and Dario Livio Longo).
-
12.
Noninvasive Renal Perfusion Measurement Using Arterial Spin Labeling (ASL ) MRI: Basic Concepts (Min-Chi Ku, María A Fernández-Seara, Frank Kober, and Thoralf Niendorf).
-
13.
Renal pH Imaging Using Chemical Exchange Saturation Transfer (CEST )-MRI: Basic Concepts (Dario Livio Longo, Pietro Irrera, Lorena Consolino, Phillip Zhe Sun, and Michael T. McMahon).
-
14.
Sodium (23Na) MRI of the Kidney: Basic Concepts (James T. Grist, Esben Søvsø Hansen, Frank G. Zöllner, and Christoffer Laustsen).
-
15.
Hyperpolarized Carbon (13C) MRI of the Kidneys: Basic Concepts (Cornelius von Morze, Galen D. Reed, Zhen J. Wang, Michael A. Ohliger, and Christoffer Laustsen).
-
16.
Functional Imaging Using Fluorine (19F) MR Methods: Basic Concepts (Sonia Waiczies, Christian Prinz, Ludger Starke, Jason M. Millward, Paula Ramos Delgado, Jens Rosenberg, Marc Nazaré, Helmar Waiczies, Andreas Pohlmann, and Thoralf Niendorf).
-
17.
MR Elastography of the Abdomen: Basic Concepts (Suraj D. Serai and Meng Yin).
3.2.4 Part IV—Experimental Protocols
-
18.
Monitoring Renal Hemodynamics and Oxygenation by Invasive Probes : Experimental Protocol (Kathleen Cantow, Mechthild Ladwig-Wiegard, Bert Flemming, Andreas Pohlmann, Thoralf Niendorf, and Erdmann Seeliger).
-
19.
Essential Practical Steps for MRI of the Kidney in Experimental Research (Andreas Pohlmann, João dos Santos Periquito, and Thoralf Niendorf).
-
20.
Assessment of Renal Volume with MRI: Experimental Protocol (Andreas Müller and Martin Meier).
-
21.
Experimental Protocols for MRI Mapping of Renal T1 (Philippe Garteiser, Octavia Bane, Sabrina Doblas, Iris Friedli, Stefanie Hectors, Gwenaël Pagé, Bernard E. Van Beers, and John C. Waterton).
-
22.
Experimental Protocols for Mapping of Renal T2* and T2 (Andreas Pohlmann, Kaixuan Zhao, Sean B. Fain, Pottumarthi V. Prasad, and Thoralf Niendorf).
-
23.
Renal MRI Diffusion : Experimental Protocol (João S. Periquito, Martin Meier, Thoralf Niendorf, Andreas Pohlmann, and Neil Peter Jerome).
-
24.
Dynamic Contrast Enhanced (DCE ) MRI–Derived Renal Perfusion and Filtration : Experimental Protocol (Pietro Irrera, Lorena Consolino, Walter Dastrù, Michael Pedersen, Frank G. Zöllner, and Dario Livio Longo).
-
25.
Renal Blood Flow Using Arterial Spin Labeling (ASL) MRI: Experimental Protocol and Principles (Kai-Hsiang Chuang, Martin Meier, María A Fernández-Seara, Frank Kober, and Min-Chi Ku).
-
26.
Renal pH Mapping Using Chemical Exchange Saturation Transfer (CEST) MRI: Experimental Protocol (Kowsalya Devi Pavuluri, Lorena Consolino, Dario Livio Longo, Pietro Irrera, Phillip Zhe Sun, and Michael T. McMahon).
-
27.
Sodium (23Na) MRI of the Kidney: Experimental Protocol (James T. Grist, Esben Søvsø Hansen, Frank G. Zöllner, and Christoffer Laustsen).
-
28.
Hyperpolarized Carbon (13C) MRI of the Kidney: Experimental Protocol (Christoffer Laustsen, Cornelius von Morze, and Galen Reed).
-
29.
Fluorine (19F) MRI for Assessing Inflammatory Cells in the Kidney: Experimental Protocol (Min-Chi Ku, Adrian Schreiber, Paula Ramos Delgado, Philipp Boehm-Sturm, Ralph Kettritz, Thoralf Niendorf, Andreas Pohlmann, and Sonia Waiczies).
-
30.
Fluorine (19F) MRI to Measure Renal Oxygen Tension and Blood Volume : Experimental Protocol (Lingzhi Hu, Hua Pan, and Samuel A. Wickline).
-
31.
MR Elastography of the Abdomen: Experimental Protocols (Suraj D. Serai and Meng Yin).
3.2.5 Part V—Protocols for Advanced Analyses
-
32.
Subsegmentation of the Kidney in Experimental MR Images Using Morphology-Based Regions of Interest or Multiple-Layer Concentric Objects (Leili Riazy, Bastien Milani, João S. Periquito, Kathleen Cantow, Thoralf Niendorf, Menno Pruijm, Erdmann Seeliger, and Andreas Pohlmann).
-
33.
Denoising for Improved Parametric MRI of the Kidney: Protocol for Nonlocal Means Filtering (Ludger Starke, Karsten Tabelow, Thoralf Niendorf, and Andreas Pohlmann).
-
34.
Analysis Protocols for MRI Mapping of Renal T1 (Philippe Garteiser, Gwenaël Pagé, Sabrina Doblas, Octavia Bane, Stefanie Hectors, Iris Friedli, Bernard E. Van Beers, and John C. Waterton).
-
35.
Analysis Protocols for MRI Mapping of the Blood Oxygenation Sensitive Parameters T2* and T2 in the Kidney (João S. Periquito, Ludger Starke, Carlota M. Santos, Andreia C. Freitas, Nuno Loução, Pablo García Polo, Rita G. Nunes, Thoralf Niendorf, and Andreas Pohlmann).
-
36.
Analysis of Renal Diffusion Weighted Imaging (DWI ) Using Apparent Diffusion Coefficient (ADC) and Intravoxel Incoherent Motion (IVIM ) Models (Neil Peter Jerome and João S. Periquito).
-
37.
Analysis Protocol for Dynamic Contrast Enhanced (DCE ) MRI of Renal Perfusion and Filtration (Frank G. Zöllner, Walter Dastrù, Pietro Irrera, Dario Livio Longo, Kevin M Bennett, Scott C. Beeman, G. Larry Bretthorst, and Joel R. Garbow).
-
38.
Quantitative Analysis of Renal Perfusion by Arterial Spin Labeling (Kai-Hsiang Chuang, Frank Kober, and Min-Chi Ku).
-
39.
Analysis Protocol for the Quantification of Renal pH Using Chemical Exchange Saturation Transfer (CEST) MRI (Hahnsung Kim, Yin Wu, Daisy Villano, Dario Livio Longo, Michael T. McMahon, and Phillip Zhe Sun).
-
40.
Analysis Protocol for Renal Sodium (23Na) MR Imaging (James T. Grist, Esben Søvsø Hansen, Frank G. Zöllner, and Christoffer Laustsen).
-
41.
Analysis Methods for Hyperpolarized Carbon (13C) MRI of the Kidney (Galen D. Reed, Natalie J. Korn, Christoffer Laustsen, and Cornelius von Morze).
-
42.
Data Preparation Protocol for Low Signal-to-Noise Ratio Fluorine-19 MRI (Ludger Starke, Thoralf Niendorf, and Sonia Waiczies).
3.3 Mission and Vision
The mission of this book was to bring together in one collection a comprehensive assortment of protocols, methods, techniques, and recommendations that can form a cornerstone of preclinical renal MR. This collection will serve as an excellent starting point for new researchers interested in breaking into the renal MR field. Expanding the number of researchers is absolutely critical for realizing the full potential of renal MR. By providing a set of carefully constructed protocols, we can avoid the waste of time, money and resources by no longer “re-inventing the wheel.” This collection will also greatly facilitate the harmonization of studies, and promote the sharing of data and results across multiple research groups, by getting everyone onto the “same page.” These efforts will help us break through those bottlenecks of inefficient learning and lack of standardization. The road ahead to fully realize the scientific and clinical possibilities of renal MR remains long, but the end result will be well worth the effort.
4 Notes
-
1.
The search string for clinical renal MRI was as follows: (((“magnetic resonance imaging”[MeSH Terms]) AND “kidney”[MeSH Terms]) AND “humans “[MeSH Terms]). For preclinical renal MRI the last AND term was replaced with AND “animals”[MeSH Terms]) NOT “humans”[MeSH Terms]. For cardiac MRI the word “kidney” was replaced with the word “heart.” For restricting the search to papers with a main focus on MRI of the kidney/heart MeSH Terms was replaced with MeSH Major Topic: (((“magnetic resonance imaging”[MeSH Major Topic]) AND “kidney”[MeSH Major Topic])) …. We used PubMed’s MeSH terms rather than free text search in the title/abstract to reduce the number of false positive/negative search results. However, a drawback of using MeSH terms is that the indexing process takes rather long (several studies reported average delays of 100–150 days), but it may take significantly longer in some cases. For example, we found that several of our publications from 2017 and after were not yet MeSH indexed. Hence, the presented publication statistics can only be rough estimates of the true numbers of publications. Data for the year 2019 was excluded, because most PubMed listed publications on renal MRI had not been indexed with MeSH Terms yet.
-
2.
We further exploited the publication lists obtained from the above PubMed’s searches. We defined active researchers as anyone who published on renal MRI since 2015. After exporting the date-restricted publications lists from PubMed in XML format, we used an in-house developed software to extract the affiliations of the first and last authors—these research groups (departments of hospitals and academic institutions were counted individually) were considered to be active players in renal MRI. Duplicates were removed automatically (using Matlab’s unique() function) and manually. The limitations mentioned in Note 1 also apply here: due to the incomplete MeSH indexing the derived statistics are only rough estimates and data of 2019 could not be included.
References
Dickerson EC, Dillman JR, Smith EA, DiPietro MA, Lebowitz RL, Darge K (2015) Pediatric MR urography: indications, techniques, and approach to review. Radiographics 35(4):1208–1230. https://doi.org/10.1148/rg.2015140223
Khrichenko D, Darge K (2010) Functional analysis in MR urography—made simple. Pediatr Radiol 40(2):182–199. https://doi.org/10.1007/s00247-009-1458-4
National Kidney Foundation NY, USA. https://www.kidney.org/professionals/kdoqi/gfr_calculator. Accessed 07 Jan 2020
Porrini E, Ruggenenti P, Luis-Lima S, Carrara F, Jimenez A, de Vries APJ, Torres A, Gaspari F, Remuzzi G (2019) Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 15(3):177–190. https://doi.org/10.1038/s41581-018-0080-9
Friedli I, Crowe LA, Berchtold L, Moll S, Hadaya K, de Perrot T, Vesin C, Martin PY, de Seigneux S, Vallee JP (2016) New magnetic resonance imaging index for renal fibrosis assessment: a comparison between diffusion-weighted imaging and T1 mapping with histological validation. Sci Rep 6:30088. https://doi.org/10.1038/srep30088
Pohlmann A, Arakelyan K, Hentschel J, Cantow K, Flemming B, Ladwig M, Waiczies S, Seeliger E, Niendorf T (2014) Detailing the relation between renal T2* and renal tissue pO2 using an integrated approach of parametric magnetic resonance imaging and invasive physiological measurements. Investig Radiol 49(8):547–560. https://doi.org/10.1097/RLI.0000000000000054
SCMR (2018) The Society for Cardiovascular Magnetic Resonance—News 2018. scmr.org/page/CMR2018news. Accessed 07 Jan 2020
Pohlmann A, Seeliger E, Grosenick D, Waiczies S, Cantow K, Persson PB, Niendorf T (2017) 2nd International Scientific Symposium on Functional Renal Imaging: Where Physiology, Nephrology, Radiology and Physics Meet. www.mdc-berlin.de/renal. Accessed 07 Feb 2020
Francis S, Taal M, Selby N (2019) 3rd International Symposium on Functional Renal Imaging. www.nottingham.ac.uk/go/3rdrenalmri. Accessed 07 Feb 2020
SpringerNature Springer Protocols. www.springernature.com/gp/librarians/products/product-types/database/springerprotocols. Accessed 10 Feb 2020
Dalkey N, Helmer O (1963) An experimental application of the DELPHI method to the use of experts. Manag Sci 9(3):458–467. https://doi.org/10.1287/mnsc.9.3.458
Mendichovszky I, Pullens P, Dekkers I, Nery F, Bane O, Pohlmann A, de Boer A, Ljimani A, Odudu A, Buchanan C, Sharma K, Laustsen C, Harteveld A, Golay X, Pedrosa I, Alsop D, Fain S, Caroli A, Prasad P, Francis S, Sigmund E, Fernandez-Seara M, Sourbron S (2020) Technical recommendations for clinical translation of renal MRI: a consensus project of the cooperation in science and technology action PARENCHIMA. MAGMA 33(1):131–140. https://doi.org/10.1007/s10334-019-00784-w
Nery F, Buchanan CE, Harteveld AA, Odudu A, Bane O, Cox EF, Derlin K, Gach HM, Golay X, Gutberlet M, Laustsen C, Ljimani A, Madhuranthakam AJ, Pedrosa I, Prasad PV, Robson PM, Sharma K, Sourbron S, Taso M, Thomas DL, Wang DJJ, Zhang JL, Alsop DC, Fain SB, Francis ST, Fernandez-Seara MA (2020) Consensus-based technical recommendations for clinical translation of renal ASL MRI. MAGMA 33(1):1–21. https://doi.org/10.1007/s10334-019-00800-z
Bane O, Mendichovszky IA, Milani B, Dekkers IA, Deux JF, Eckerbom P, Grenier N, Hall ME, Inoue T, Laustsen C, Lerman LO, Liu C, Morrell G, Pedersen M, Pruijm M, Sadowski EA, Seeliger E, Sharma K, Thoeny H, Vermathen P, Wang ZJ, Serafin Z, Zhang JL, Francis ST, Sourbron S, Pohlmann A, Fain SB, Prasad PV (2020) Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA 33(1):199–215. https://doi.org/10.1007/s10334-019-00802-x
Ljimani A, Caroli A, Laustsen C, Francis S, Mendichovszky IA, Bane O, Nery F, Sharma K, Pohlmann A, Dekkers IA, Vallee JP, Derlin K, Notohamiprodjo M, Lim RP, Palmucci S, Serai SD, Periquito J, Wang ZJ, Froeling M, Thoeny HC, Prasad P, Schneider M, Niendorf T, Pullens P, Sourbron S, Sigmund EE (2020) Consensus-based technical recommendations for clinical translation of renal diffusion-weighted MRI. MAGMA 33(1):177–195. https://doi.org/10.1007/s10334-019-00790-y
Dekkers IA, de Boer A, Sharma K, Cox EF, Lamb HJ, Buckley DL, Bane O, Morris DM, Prasad PV, Semple SIK, Gillis KA, Hockings P, Buchanan C, Wolf M, Laustsen C, Leiner T, Haddock B, Hoogduin JM, Pullens P, Sourbron S, Francis S (2020) Consensus-based technical recommendations for clinical translation of renal T1 and T2 mapping MRI. MAGMA 33(1):163–176. https://doi.org/10.1007/s10334-019-00797-5
Acknowledgments
This work was funded, in part (Thoralf Niendorf, Sonia Waiczies, Andreas Pohlmann, Erdmann Seeliger and Joao Periquito), by the German Research Foundation (Gefoerdert durch die Deutsche Forschungsgemeinschaft (DFG), Projektnummer 394046635, SFB 1365, RENOPROTECTION. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Project number 394046635, SFB 1365, RENOPROTECTION).
This chapter is based upon the work from COST Action PARENCHIMA, supported by European Cooperation in Science and Technology (COST). COST (www.cost.eu) is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to enrich their ideas by sharing them with their peers. This boosts their research, career, and innovation.
PARENCHIMA (renalmri.org) is a community-driven Action in the COST program of the European Union, which unites more than 200 experts in renal MRI from 30 countries with the aim to improve the reproducibility and standardization of renal MRI biomarkers.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Pohlmann, A. et al. (2021). Recommendations for Preclinical Renal MRI: A Comprehensive Open-Access Protocol Collection to Improve Training, Reproducibility, and Comparability of Studies. In: Pohlmann, A., Niendorf, T. (eds) Preclinical MRI of the Kidney. Methods in Molecular Biology, vol 2216. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0978-1_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0978-1_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0977-4
Online ISBN: 978-1-0716-0978-1
eBook Packages: Springer Protocols