Abstract
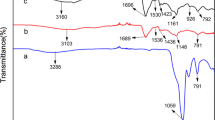
The present manuscript deals with the synthesis of novel chitosan-based semi-interpenetrating networks (semi-CIPNs) and explores their potential for removal of Cd2+ ions from solution. Microwave radiation-induced polymerization was carried out using biopolymer chitosan (CS) and acrylic acid (AA) monomer, in the presence of initiator (K2S2O8) and cross-linker thiourea (CH4N2S). The effect of polymerization variables such as the amount of solvent, concentrations of initiator, monomer and cross-linker and the reaction time were optimized as a function of percentage grafting (Pg). Under optimized conditions, the liquid uptake potential of the synthesized semi-CIPN was studied in terms of percentage swelling (Ps). The semi-CIPN showed the maximum percentage grafting (3845%) and percentage swelling (311%) under the optimized conditions. The physico-chemical techniques such as Fourier transform infrared spectroscopy (FTIR), thermal analysis (TGA/DTG/DTA), scanning electron microscopy (SEM), and X-ray diffractometry (XRD) provided the evidence for formation of semi-CIPN from chitosan. Semi-CIPN was found to be an efficient device for facile sequestering of Cd2+ ions on the basis of batch experimental studies as 98.1% efficiency was observed for removal of Cd2+ from 100 mg/L solution. Reusability of polymeric material was evaluated for its sorption performance in consecutive adsorption and desorption cycle and it was observed that the regeneration efficiency decreases slowly up to eighth adsorption/desorption cycles, thereafter, it decreases significantly in the 9th and 10th cycles and 40% efficiency has been observed after 12th cycle. Regeneration of the synthesized semi-CIPN explores the reusability and economic feasibility of biopolymer material.

















Similar content being viewed by others
References
Oller I, Malato S, Sánchez-Pérez J (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination: a review. Sci Total Environ 409:4141–4166
Wang H, Ren ZJ (2014) Bioelectrochemical metal recovery from wastewater: a review. Water Res 66:219–232
Park JY, Yoo YJ (2009) Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose. Appl Microbiol Biotechnol 82:415–429
Kurniawan TA, Chan GY, Lo WH, Babel S (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling: an overview. Rsc Adv 2:6380–6388
Hu H (2002) Human health and heavy metals life support: the environment and human health. MIT Press, Cambridge
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79:241–244
MatisKA ZouboulisAI, Hancock IC (1994) Waste microbial biomass for cadmium ion removal: application of flotation for downstream separation. Bioresour Techno l49:253–259
Ramachandra TV, Sudarshan PB, Mahesh MK,VinayS (2018) Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J Environ Manage 206:1204–1210
Çelik S, Akar ST, Şölener M, Akar T (2017) Anionically reinforced hydrogel network entrapped fungal cells for retention of cadmium in the contaminated aquatic media. J Environ Manage 204:583–593
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Hubbe MA, Hasan SH, Ducoste JJ (2011) Cellulosic substrates for removal of pollutants from aqueous systems: A review 1 Metals. Bio Resour 6:2161–2287
Yeul VS, Rayalu SS (2013) Unprecedented chitin and chitosan: a chemical overview. J Polym Environ 21:606–614
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43:401–414
Mourya VK, Inamdar NN (2008) Chitosan-modifications and applications: opportunities galore. React Funct Polym 68:1013–1051
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Kołodyńska D (2012) Adsorption characteristics of chitosan modified by chelating agents of a new generation. Chem Eng J 179:33–43
Miretzky P, Cirelli AF (2009) Hg (II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167:10–23
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Manson JA (2012) Polymer blends and composites. Springer Science & Business Media, New York
Jing G, Wang L, Yu H, Amer WA, Zhang L (2013) Recent progress on study of hybrid hydrogels for water treatment. Colloids Surfac A Physicochem Eng Aspts 416:86–94
Garg K, Kaur MP, Sud D (2008) Removal of nickel [II] from aqueous solution by adsorption on agricultural waste biomass using a response methodological approach. Bioresour Technol 99:1325–1331
Mahajan G, Garg U, Sud D, Garg V (2013) Utilization Properties of Jatropha De-oiled cake for removal of Nickel (ii) from aqueous solutions. Bio Resour 8:5596–5611
Kumar K, Kaith BS, Mittal H (2012) A study on effect of different reaction conditions on grafting of psyllium and acrylic acid-based hydrogels. J Appl Polym Sci 123:1874–1883
Maiti M, Jindal R, Kaith BS, Jana AK (2011) Synthesis of graft copolymers of binary vinyl monomer mixtures onto acetylated Saccharum spontaneum L and characterization. J Appl Polym Sci 121:2060–2071
BhullarN KumariK, Sud D (2018) A biopolymer-based composite hydrogel for rhodamine 6G dye removal: its synthesis, adsorption isotherms and kinetics. Iran Polym J l27:527–535
Kweon H, Um IC, Park YH (2001) Structural and thermal characteristics of Antheraea pernyi silk fibroin/chitosan blend film. Polymer 42:6651–6656
Demirbaş O, Alkan M, Doğan M (2002) The removal of Victoria blue from aqueous solution by adsorption on a low-cost material. Adsorption 8:341–349
Shukla SS, Yu LJ, Dorris KL, Shukla A (2005) Removal of nickel from aqueous solutions by sawdust. J Hazard Mater 121:243–246
Liu HL, Chen BY, Lan YW, Cheng YC (2004) Biosorption of Zn (II) and Cu (II) by the indigenous Thio bacillus thio oxidans. Chem Eng J 97:195–201
Acknowledgements
This research project could not have been accomplished without the support of Sant Longowal Institute of Engineering and Technology, Longowal. We are pleased to acknowledge the facilities provided by National Institute of Technology Jalandhar and Panjab University, Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This project is self-financed and the authors have no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Bhullar, N.K., Kumari, K. & Sud, D. Semi-interpenetrating networks of biopolymer chitosan/acrylic acid and thiourea hydrogels: synthesis, characterization and their potential for removal of cadmium. Iran Polym J 28, 225–236 (2019). https://doi.org/10.1007/s13726-019-00693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-019-00693-8