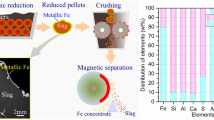
Abstract
In this paper, a novel process for beneficiation of metallic iron from nickel leaching residue and preparation of ceramsite from tailings by direct reduction–magnetic separation process is reported. The optimal conditions for direct reduction process were 1100 °C roasting temperature, 120 min duration, and 30 wt.% reductant dosage. The reduced sample was benefited from low-intensity magnetic separation. This process yielded an iron concentrate of 82.32 wt.% grade and 78.05 wt.% recovery. Hence, this metallic iron could be used as a feedstock for the steel industry. Tailings of the magnetic separation procedure were used to prepare ceramsite. Optimal conditions for preparing ceramsite were: 55% magnetic separation tailings, 20% silica, 15% fly ash, 10% charcoal, a 1150 °C roasting temperature, and a holding time of 30 min. The ceramsite properties met the requirement of CJ/T299-2008 National Standard. These results suggested that developing this solid waste would have environmental and economic benefits.















Similar content being viewed by others
References
Valix M, Tang J Y, and Cheung W H, Miner Eng J 12 (2001)1629.
Guo Q, Qu J K, Han B B, Zhang P Y, Song Y X, and Qi T, Miner Eng J 2 (2015)1.
Ma B Z, Wang C Y, Yang W J, Yin F, and Chen Y Q, Miner Eng J 9 (2013)107.
Ma B Z, Wang C Y, Yang W J, Yang B, and Zhang Y L, Miner Eng J 5 (2013)152.
Elliott R, and Pickles C A, High Temp Mater Processes J 9 (2017) 836.
Loveday B K, Miner Eng J 7 (2008)534.
Macasek F, Kufcakova J, Rajec P, Kopunec R, Jakabsky S, Lovas M, and Hredzak S, Chem Papers-Chemicke Zvesti J 3 (2004)163.
Zhu D Q, Zhou X L, Luo Y H, Pan J, and Bai B, High Temp Mater Processes J 10 (2016)1031.
Dasgupta R, and Chaubey A K, Trans Indian Inst Met J 6 (2009)187.
Whittington B I and Johnson J A, Hydrometall J, 78 (2005) 256.
Stamboliadis E, Alevizos G, and Zafiratos J, Miner Eng J 17 (2004) 245.
Dorfling C, Akdogan G, Bradshaw S M, and Eksteen J J, Miner Eng J 6 (2011)583.
Wang R, Liu Z G, Chu M S, Wang H T, Zhao W, and Gao L H, J Iron Steel Res J 5 (2018)497.
Zhu D Q, Luo Y H, Pan J, and Zhou X L, High Temp Mater Processes J 2 (2016)187.
Yu W, Sun T C, Liu Z Z, Kou J, and Xu C Y, ISIJ Int J 1 (2014)56.
Yu W, Wen X J, Chen J G, Kuang J Z, Tang Q Y, Tian Y C, Fu J L, Huang W Q, and Qiu T S, Miner J 2 (2017)2.
Guo Z Q, Zhu D Q, Pan J, Yao W J, Xu W Q, and Chen J, JOM J 9 (2017) 1688.
Zhou X L, Zhu D Q, Pan J, and Wu T J, ISIJ Int J 7 (2015)1347.
Lei C, Yan B, Chen T, and Xiao X M, J Clean Prod J 8 (2017) 74.
Li C, Sun H H, Bai J, and Li L T, J Hazard Mater J 2 (2010) 71.
Yang C C, Li S Q, Zhang C Q, Bai J X, and Guo Z J, Miner Process Extr Metall Rev J 1 (2018)44.
Kumar R, Das P, Beulah M, Arjun H R, and Ignaitus G, J Adv Manuf Syst J 3 (2017)276.
Gayana B C and Chandar K R, Adv Concr Constr J 3 (2018) 222.
Manjarrez L, and Zhang L Y, J Mater Civ Eng J 9 (2018) 33.
Chen Q S, Zhang Q L, Fourie A, and Xin C, J Environ Manag J 10 (2017)20.
Kinnunen P, Ismailov A, Solismaa S, Sreenivasan H, Räisänen M L, Levänen E, and Illikainen M, J Clean Prod J 10 (2017)635.
Wu H Q, Zhang T, Pan R J, Chun Y Y, Zhou H M, Zhu W X, Peng H Z, and Zhang Q, Constr Build Mater J 5 (2018)368.
Sun M T, Yang Z M, Lu J, Fan X L, Guo R B, and Fu S F, J Chem Technol Biotechnol 2 (2018)2408.
Li H, Guo Z, Wu D F, Fan J, Huang S B, and Zhou S F, Mater J 3 (2018)359.
Wen S H, Chen L, Li W Q, Ren H Q, Li K, Wu B, Hu H D, and Xu K, Sci Rep J 6 (2018)2.
Jing Q X, Wang Y Y, Chai L Y, Tang C J, Huang X D, Guo H, Wang W, and You W, Trans Nonferrous Met Soc China J 5 (2018)1053.
Liu G S, Strezov V, Lucas J A, and Wibberley L J, Thermochim Acta J 410 (2004)133.
Acknowledgements
The authors wish to express their thanks to the Natural Science Foundation of China (NO.5157041410) for the financial support of this research.
Author information
Authors and Affiliations
Contributions
Qiang Zhao conducted the experimental work and prepared the manuscript; Jilai Xue directed the research work and modified the manuscript; Wen Chen participated in the design of the research work at different stages.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, Q., Xue, J. & Chen, W. Zero-Waste Recycling Method for Nickel Leaching Residue by Direct Reduction–Magnetic Separation Process and Ceramsite Preparation. Trans Indian Inst Met 72, 1075–1085 (2019). https://doi.org/10.1007/s12666-019-01582-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-019-01582-7