Abstract
Background
The mammalian target of rapamycin complex 1 (mTORC1) is fundamental for many cellular processes, yet it is often dysregulated with aging. Increased amino acid (AA) availability is correlated with the expression of AA transporters (AAT) and mTORC1 activity. Although many AA sensors and mediators have been proposed to relay the AA signal to mTORC1, it has not yet been determined if chronic dietary intervention affects the expression of AAT, sensors and mediators and their relationships with mTORC1 activity.
Objective and Design
This study investigated whether the consumption of a diet containing either the current recommended daily allowance (RDA) of protein intake (0.8 g/kg/d) or twice the RDA (2RDA) for ten weeks affected the expression of targets associated with AA transport, sensing and mTORC1 regulation in 26 older men (70-81 years).
Method
Muscle biopsies were collected before and after the intervention under fasting conditions. Diets were controlled by providing fully prepared meals and snacks. Western blot and quantitative polymerase chain reaction were used to measure protein and gene expression respectively.
Results
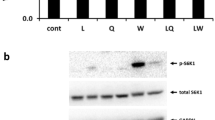
Consumption of 2RDA reduced the protein expression of L-type amino acid transporter 1 (LAT1). However, plasma leucine concentration and basal mTORC1 activity were unaltered. The downregulation of LAT1 did not affect the expression of AA sensors and mediators, including leucyl tRNA synthetase (LRS), cytosolic arginine sensor for mTORC1 (CASTOR1), Sestrin2 and Rag proteins. Instead, total ribosomal protein S6 (RPS6) was upregulated with 2RDA.
Conclusion
Ten weeks of 2RDA diet did not affect the fasting mTORC1 signaling, but increased total RPS6 might suggest improved muscular translational capacity to maintain muscular mass.




Similar content being viewed by others
References
Wolfe, RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–482.
Goodpaster, BH, Park, SW, Harris, TB, Kritchevsky, SB, Nevitt, M, Schwartz, AV, Simonsick, EM, Tylavsky, FA, Visser, M, Newman, AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064.
Janssen, I, Heymsfield, SB, Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896.
Srikanthan, P, Karlamangla, AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. The Journal of Clinical Endocrinology & Metabolism 2011;96:2898–2903.
Visser, M, Goodpaster, BH, Kritchevsky, SB, Newman, AB, Nevitt, M, Rubin, SM, Simonsick, EM, Harris, TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2005;60:324–333.
Paddon-Jones, D, Rasmussen, BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90.
Mitchell, CJ, Milan, AM, Mitchell, SM, Zeng, N, Ramzan, F, Sharma, P, Knowles, SO, Roy, NC, Sjodin, A, Wagner KH et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr 2017;106:1375–1383.
McAuley, K, Hopkins, C, Smith, K, McLay, R, Williams, S, Taylor, R, Mann, J. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulinresistant obese women. Diabetologia 2005;48:8–16.
Remond, D, Machebeuf, M, Yven, C, Buffiere, C, Mioche, L, Mosoni, L, Patureau Mirand, P. Postprandial whole-body protein metabolism after a meat meal is influenced by chewing efficiency in elderly subjects. Am J Clin Nutr 2007;85:1286–1292.
Donini, LM, Savina, C, Cannella, C. Eating habits and appetite control in the elderly: the anorexia of aging. International psychogeriatrics 2003;15:73–87.
Milan, A, D’souza, R, Pundir, S, Pileggi, C, Barnett, G, Markworth, J, Cameron-Smith, D, Mitchell, C. Older adults have delayed amino acid absorption after a high protein mixed breakfast meal. J Nutr Health Aging 2015;19:839.
van der Zanden Lotte, DT, van Kleef, E, de Wijk, RA, van Trijp, HC. Knowledge, perceptions and preferences of elderly regarding protein-enriched functional food. Appetite 2014;80:16–22.
Barton, A, Beigg, C, Macdonald, I, Allison, S. High food wastage and low nutritional intakes in hospital patients. Clinical Nutrition 2000;19:445–449.
World Health Organization, United Nations University. Protein and Amino Acid Requirements in Human Nutrition., World Health Organization, 2007.
US Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans. Washington (DC), USDA, 2015.
Ministry of Health. Food and Nutrition Guidelines for Healthy Older People: A Background Paper. Wellington, Ministry of Health, 2013.
Houston, DK, Nicklas, BJ, Ding, J, Harris, TB, Tylavsky, FA, Newman, AB, Lee, JS, Sahyoun, NR, Visser, M, Kritchevsky SB et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–155.
Dreyer, HC, Drummond, MJ, Pennings, B, Fujita, S, Glynn, EL, Chinkes, DL, Dhanani, S, Volpi, E, Rasmussen, BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294:E392–400.
Dickinson, JM, Fry, CS, Drummond, MJ, Gundermann, DM, Walker, DK, Glynn, EL, Timmerman, KL, Dhanani, S, Volpi, E, Rasmussen, BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 2011;141:856–862.
Laplante, M, Sabatini, DM. mTOR signaling in growth control and disease. Cell 2012;149:274–293.
Markofski, MM, Dickinson, JM, Drummond, MJ, Fry, CS, Fujita, S, Gundermann, DM, Glynn, EL, Jennings, K, Paddon-Jones, D, Reidy, PT. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol 2015;65:1–7.
Kimball, SR, O’Malley, JP, Anthony, JC, Crozier, SJ, Jefferson, LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. American Journal of Physiology-Endocrinology and Metabolism 2004;287:E772–E780.
Sandri, M, Barberi, L, Bijlsma, A, Blaauw, B, Dyar, K, Milan, G, Mammucari, C, Meskers, C, Pallafacchina, G, Paoli, A. Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013;14:303–323.
Paturi, S, Gutta, AK, Katta, A, Kakarla, SK, Arvapalli, RK, Gadde, MK, Nalabotu, SK, Rice, KM, Wu, M, Blough, E. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech Ageing Dev 2010;131:202–209.
Johnson, SC, Rabinovitch, PS, Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013;493:338–345.
Menon, S, Yecies, JL, Zhang, HH, Howell, JJ, Nicholatos, J, Harputlugil, E, Bronson, RT, Kwiatkowski, DJ, Manning, BD. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal 2012;5:ra24.
Glynn, EL, Fry, CS, Drummond, MJ, Timmerman, KL, Dhanani, S, Volpi, E, Rasmussen, BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–1976.
Hundal, HS, Taylor, PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 2009;296:E603–13.
Taylor, PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr 2014;99:223S-230S.
Dickinson, JM, Gundermann, DM, Walker, DK, Reidy, PT, Borack, MS, Drummond, MJ, Arora, M, Volpi, E, Rasmussen, BB. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr 2014;144:1694–1702.
Reidy, PT, Walker, DK, Dickinson, JM, Gundermann, DM, Drummond, MJ, Timmerman, KL, Cope, MB, Mukherjea, R, Jennings, K, Volpi E et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol 2014;116:1353–1364.
Pasiakos, SM, Cao, JJ, Margolis, LM, Sauter, ER, Whigham, LD, McClung, JP, Rood, JC, Carbone, JW, Combs, GF,Jr, Young, AJ. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J 2013;27:3837–3847.
Bar-Peled, L, Schweitzer, LD, Zoncu, R, Sabatini, DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012;150:1196–1208.
Sancak, Y, Peterson, TR, Shaul, YD, Lindquist, RA, Thoreen, CC, Bar-Peled, L, Sabatini, DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008;320:1496–1501.
Han, JM, Jeong, SJ, Park, MC, Kim, G, Kwon, NH, Kim, HK, Ha, SH, Ryu, SH, Kim, S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–424.
Bonfils, G, Jaquenoud, M, Bontron, S, Ostrowicz, C, Ungermann, C, De Virgilio, C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 2012;46:105–110.
Wang, S, Tsun, ZY, Wolfson, RL, Shen, K, Wyant, GA, Plovanich, ME, Yuan, ED, Jones, TD, Chantranupong, L, Comb W et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015;347:188–194.
Rebsamen, M, Pochini, L, Stasyk, T,–. Araújo, ME, Galluccio, M, Kandasamy, RK, Snijder, B, Fauster, A, Rudashevskaya, EL, Bruckner, M. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015;519:477–481.
Jung, J, Genau, HM, Behrends, C. Amino acid dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol Cell Biol 2015;35:2479–2494.
Bos, JL, Rehmann, H, Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007;129:865–877.
Bar-Peled, L, Chantranupong, L, Cherniack, AD, Chen, WW, Ottina, KA, Grabiner, BC, Spear, ED, Carter, SL, Meyerson, M, Sabatini, DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 2013;340:1100–1106.
Chantranupong, L, Scaria, SM, Saxton, RA, Gygi, MP, Shen, K, Wyant, GA, Wang, T, Harper, JW, Gygi, SP, Sabatini, DM. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 2016;165:153–164.
Saxton, RA, Chantranupong, L, Knockenhauer, KE, Schwartz, TU, Sabatini, DM. Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature 2016;536:229–233.
Wolfson, RL, Chantranupong, L, Saxton, RA, Shen, K, Scaria, SM, Cantor, JR, Sabatini, DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016;351:43–48.
Parmigiani, A, Nourbakhsh, A, Ding, B, Wang, W, Kim, YC, Akopiants, K, Guan, K, Karin, M, Budanov, AV. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell reports 2014;9:1281–1291.
Churchward-Venne, TA, Burd, NA, Mitchell, CJ, West, DW, Philp, A, Marcotte, GR, Baker, SK, Baar, K, Phillips, SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 2012;590:2751–2765.
Drummond, MJ, Glynn, EL, Fry, CS, Timmerman, KL, Volpi, E, Rasmussen, BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8.
Mitchell, CJ, McGregor, RA, D’Souza, RF, Thorstensen, EB, Markworth, JF, Fanning, AC, Poppitt, SD, Cameron-Smith, D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 2015;7:8685–8699.
Ye, J, Coulouris, G, Zaretskaya, I, Cutcutache, I, Rozen, S, Madden, TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012;13:134.
Livak, KJ, Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001;25:402–408.
Vandesompele, J, De Preter, K, Pattyn, F, Poppe, B, Van Roy, N, De Paepe, A, Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:research0034.1.
Eisenberg, E, Levanon, EY. Human housekeeping genes, revisited. Trends in Genetics 2013;29:569–574.
Drummond, MJ, Dickinson, JM, Fry, CS, Walker, DK, Gundermann, DM, Reidy, PT, Timmerman, KL, Markofski, MM, Paddon-Jones, D, Rasmussen BB et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–22.
Knuiman, P, Hopman, M, Wouters, J, Mensink, M. Select skeletal muscle mRNAs related to exercise adaptation are minimally affected by different pre-exercise meals that differ in macronutrient profile. Frontiers in physiology 2018;9:28.
D’Souza, RF, Marworth, JF, Figueiredo, VC, Della Gatta, PA, Petersen, AC, Mitchell, CJ, Cameron-Smith, D. Dose-dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol Rep 2014;2(8).
Mitchell, CJ, Zeng, N, D’Souza, RF, Mitchell, SM, Aasen, K, Fanning, AC, Poppitt, SD, Cameron-Smith, D. Minimal dose of milk protein concentrate to enhance the anabolic signalling response to a single bout of resistance exercise; a randomised controlled trial. Journal of the International Society of Sports Nutrition 2017;14:17.
Suryawan, A, Nguyen, HV, Almonaci, RD, Davis, TA. Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids 2013;45:523–530.
Raue, U, Trappe, TA, Estrem, ST, Qian, H, Helvering, LM, Smith, RC, Trappe, S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 2012;112:1625–1636.
Wolfe, RR. Regulation of muscle protein by amino acids. J Nutr 2002;132:3219S-24S.
Shaham, O, Wei, R, Wang, TJ, Ricciardi, C, Lewis, GD, Vasan, RS, Carr, SA, Thadhani, R, Gerszten, RE, Mootha, VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol 2008;4:214.
Wurtz, P, Soininen, P, Kangas, AJ, Ronnemaa, T, Lehtimaki, T, Kahonen, M, Viikari, JS, Raitakari, OT, Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013;36:648–655.
Newgard, CB, An, J, Bain, JR, Muehlbauer, MJ, Stevens, RD, Lien, LF, Haqq, AM, Shah, SH, Arlotto, M, Slentz, CA. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell metabolism 2009;9:311–326.
Frid, AH, Nilsson, M, Holst, JJ, Björck, IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects–. Am J Clin Nutr 2005;82:69–75.
Haufe, S, Engeli, S, Kaminski, J, Witt, H, Rein, D, Kamlage, B, Utz, W, Fuhrmann, JC, Haas, V, Mahler A et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr Metab Cardiovasc Dis 2017;27:858–864.
Kim, I, Schutzler, SE, Azhar, G, Wolfe, RR, Ferrando, AA, Coker, RH. Short term elevation in dietary protein intake does not worsen insulin resistance or lipids in older adults with metabolic syndrome: a randomized-controlled trial. BMC nutrition 2017;3:33.
Jensen, A, Figueiredo-Larsen, M, Holm, R, Broberg, ML, Brodin, B, Nielsen, CU. PAT1 (SLC36A1) shows nuclear localization and affects growth of smooth muscle cells from rats. Am J Physiol Endocrinol Metab 2014;306:E65–74.
Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 2014;46:2787–2798.
Dodd, KM, Tee, AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 2012;302:E1329–42.
Yao, D, Mackenzie, B, Ming, H, Varoqui, H, Zhu, H, Hediger, MA, Erickson, JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem 2000;275:22790–22797.
Boll, M, Daniel, H, Gasnier, B. The SLC36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Archiv 2004;447:776–779.
Agergaard, J, Bulow, J, Jensen, JK, Reitelseder, S, Borno, A, Drummond, MJ, Schjerling, P, Holm, L. Effect of light-load resistance exercise on postprandial amino acid transporter expression in elderly men. Physiol Rep 2017;5:10.14814/phy2.13444. Epub 2017 Sep 27.
Wilson K, Walker J. Principles and Techniques of Biochemistry and Molecular Biology., Cambridge University Press, 2010.
Drummond, MJ, Fry, CS, Glynn, EL, Timmerman, KL, Dickinson, JM, Walker, DK, Gundermann, DM, Volpi, E, Rasmussen, BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–142.
D’Souza, RF, Bjørnsen, T, Zeng, N, Aasen, KM, Raastad, T, Cameron-Smith, D, Mitchell, CJ. MicroRNAs in Muscle: Characterizing the Powerlifter Phenotype. Frontiers in Physiology 2017;8:383.
Anthony, JC, Yoshizawa, F, Anthony, TG, Vary, TC, Jefferson, LS, Kimball, SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413–2419.
Hodson, N, Brown, T, Joanisse, S, Aguirre, N, West, DW, Moore, DR, Baar, K, Breen, L, Philp, A. Characterisation of L-Type Amino Acid Transporter 1 (LAT1) Expression in Human Skeletal Muscle by Immunofluorescent Microscopy. Nutrients 2017;10:23.
Yanagida, O, Kanai, Y, Chairoungdua, A, Kim, DK, Segawa, H, Nii, T, Cha, SH, Matsuo, H, Fukushima, J, Fukasawa, Y. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochimica et Biophysica Acta (BBA)-Biomembranes 2001;1514:291–302.
Beals, JW, Sukiennik, RA, Nallabelli, J, Emmons, RS, van Vliet, S, Young, JR, Ulanov, AV, Li, Z, Paluska, SA, De Lisio M et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am J Clin Nutr 2016;104:1014–1022.
White, Z, White, RB, McMahon, C, Grounds, MD, Shavlakadze, T. High mTORC1 signaling is maintained, while protein degradation pathways are perturbed in old murine skeletal muscles in the fasted state. Int J Biochem Cell Biol 2016;78:10–21.
Dungan, CM, Li, Z, Wright, DC, Williamson, DL. Hyperactive mTORC1 signaling is unaffected by metformin treatment in aged skeletal muscle. Muscle Nerve 2016;53:107–117.
Moss, T, Langlois, F, Gagnon-Kugler, T, Stefanovsky, V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cellular and molecular life sciences 2007;64:29–49.
Mieulet, V, Roceri, M, Espeillac, C, Sotiropoulos, A, Ohanna, M, Oorschot, V, Klumperman, J, Sandri, M, Pende, M. S6 kinase inactivation impairs growth and translational target phosphorylation in muscle cells maintaining proper regulation of protein turnover. Am J Physiol Cell Physiol 2007;293:C712–22.
Drummond, MJ, Dreyer, HC, Fry, CS, Glynn, EL, Rasmussen, BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374–1384.
Hannan, KM, Brandenburger, Y, Jenkins, A, Sharkey, K, Cavanaugh, A, Rothblum, L, Moss, T, Poortinga, G, McArthur, GA, Pearson RB et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol 2003;23:8862–8877.
Anthony, JC, Anthony, TG, Kimball, SR, Jefferson, LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 2001;131:856S-860S.
Haddad, F, Adams, GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 2006;100:1188–1203.
Zeng, N, D’Souza, RF, Mitchell, CJ, Cameron-Smith, D. Sestrins are differentially expressed with age in the skeletal muscle of men: A cross-sectional analysis. Exp Gerontol 2018;110:23–34.
Millward, D, Garlick, P, James, W, Nnanyelugo, D, Ryatt, J. Relationship between protein synthesis and RNA content in skeletal muscle. Nature 1973;241:204.
Bar-Peled, L, Sabatini, DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014;24:400–406.
Chantranupong, L, Wolfson, RL, Sabatini, DM. Nutrient-sensing mechanisms across evolution. Cell 2015;161:67–83.
Lee, JH, Cho, US, Karin, M. Sestrin regulation of TORC1: Is Sestrin a leucine sensor? Sci Signal 2016;9:re5.
Saxton RA, Knockenhauer KE, Schwartz TU, Sabatini DM. The apo-structure of the leucine sensor Sestrin2 is still elusive. Sci Signal 2016;9:ra92.
Zeng, N, D’Souza, RF, Sorrenson, B, Merry, TL, Barnett, MP, Mitchell, CJ, Cameron-Smith, D. The putative leucine sensor Sestrin2 is hyperphosphorylated by acute resistance exercise but not protein ingestion in human skeletal muscle. Eur J Appl Physiol 2018:1–13.
Carlin, MB, Tanner, RE, Agergaard, J, Jalili, T, McClain, DA, Drummond, MJ. Skeletal muscle Ras-related GTP binding B mRNA and protein expression is increased after essential amino acid ingestion in healthy humans. J Nutr 2014;144:1409–1414.
Rasmussen, BB, Volpi, E, Borack, MS, Reidy, PT, Graber, TG. Essential amino acid ingestion alters expression of genes associated with amino acid sensing, transport, and mTORC1 regulation in human skeletal muscle. Nutrition & metabolism 2017;14:35.
Zeng, N, D’Souza, R, Figueiredo, V, Markworth, J, Roberts, L, Peake, J, Mitchell, C, Cameron-Smith, D. Acute resistance exercise induces Sestrin2 phosphorylation and p62 dephosphorylation in human skeletal muscle. Physiological Reports 2017;5:e13526.
Saxton, RA, Sabatini, DM. mTOR signaling in growth, metabolism, and disease. Cell 2017;168:960–976.
Kim, JS, Ro, S, Kim, M, Park, H, Semple, IA, Park, H, Cho, U, Wang, W, Guan, K, Karin, M. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Scientific reports 2015;5:9502.
Dickinson, JM, Drummond, MJ, Coben, JR, Volpi, E, Rasmussen, BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clinical Nutrition 2013;32:273–280.
Mitchell, CJ, D’Souza, RF, Zeng, N, McGregor, RA, Fanning, AC, Poppitt, SD, Cameron-Smith, D. Understanding the sensitivity of muscle protein synthesis to dairy protein in middle-aged men. Int Dairy J 2016;63:35–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, N., Prodhan, U., d’Souza, R.F. et al. Regulation of Amino Acid Transporters and Sensors in Response to a High protein Diet: A Randomized Controlled Trial in Elderly Men. J Nutr Health Aging 23, 354–363 (2019). https://doi.org/10.1007/s12603-019-1171-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-019-1171-4