Abstract
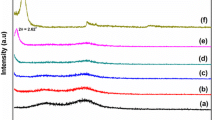
Silica–polystyrene (Si–PS) nanocomposite latex particles were prepared by emulsion polymerization using Hwangtoh clay as the silica source and blending with polyethylene terephthalate (PET) by melt extrusion. The Hwangtoh clay was mechanically grounded as nanoscale. XRD measurements showed more crystallized Hwangtoh clay in nano-dimension than that of raw material with crystallite sizes (t) of 108.52 and 126.7 nm, respectively. SEM (scanning electron microscope) measurements showed that the dispersed Si–PS hybrid nanocomposite had a D h of ~350 nm within the PET matrix. FTIR (Fourier transform infrared spectroscopy) measurements exhibited the characteristic absorption peaks of Si–O–Si stretching vibrations and Al–O–Si bending vibrations from both the Si–PS hybrid structure and PET/Si–PS composite. X-ray diffraction (XRD) measurements exhibited a characteristic Si diffraction peak of 2θ value at 25° and 29°, both from Si–PS hybrid nanoparticles and PET/Si–PS composites. The blending of PET with a Si–PS nanocomposite was determined by XPS (X-ray photoelectron spectroscopy) analysis, which showed three distinctive peaks representing the interatomic bonding of carbon. In XPS measurements, the decomposition of the Si 2p core peak demonstrated that the Si atom from Hwangtoh clay is composed of four chemical states such as Si0, Si+ (Si2O), Si2+ (SiO), and Si3+ (Si2O3). Our results provide evidence of the successful encapsulation of silicate from Hwangtoh clay in a PS shell, and further blending with PET by the melting extrusion method.






Similar content being viewed by others
References
Lee YH, Han SH, Kim YS (2005) Investigation of hydrophobic properties of PSII—modified EVOHG, LLEPE, and PET films. J Surf Anal 12(2):258–262
Umamaheswari S, Murali M (2013) FTIR spectroscopic study of fungal degradation of poly (ethylene terephthalate) and polystyrene foam. Elixir Chem Engg 64:19159–19164
Wand J, Huang N, Yang P, Leng YX, Sun H, Liu ZY, Chu PK (2004) The effects of amorphous carbon films deposited on polyethylene terephthalate on bacterial adhesion. Biomaterials 25:3163–3170
Ferreira L, Evangelista MB, Martins MCL, Granja PL, Esteves JL, Barbos M (2005) Improving the adhesion of poly(ethylene terephthalate) fibers to poly(hydroxyethyl methacrylate) hydrogels by ozone treatment: surface characterization and pull-out tests. Polymer 46:9840–9850
Maa CT, Chang FC (1993) In situ compatibilization of PET/PS blends through reactive copolymers. J Appl Polym Sci 49(5):913–924
Han K, Yu M (2006) Study of the preparation and properties of UV-blocking fabrics of a PET/TiO2 nanocomposite prepared by in situ polycondensation. J Appl Polym Sci 100(2):1588–1593
Müller K, Bugnicourt E, Latorre M, Jorda M, Sanz YE, Lagaron José M, Miesbauer O, Bianchin A, Hankin S, Bölz U, Pérez G, Jesdinszki M, Lindner M, Scheuerer A, Castelló S, Schmid M (2017) Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials. 7(74):1–47
Laoutid F, Ferry L, Lopez-Cuesta JM, Crespy A (2006) Flame-retardant action of red phosphorus/magnesium oxide and red phosphorus/iron oxide compositions in recycled PET. Fire Mater 30(5):343–358
Li W, Karger-Kocsis J, Schlarb AK (2009) Dispersion of TiO2 particles in PEPT/PP/TiO2 and PET/PP/PP-g-MA/TiO2 composites prepared with different blending procedures. Macromol Mater Eng 294:582–589
Cen JH, Dai CA, Chen HJ, Chien PC, Chiu WY (2007) Synthesis of nano-sized TiO2/poly(AA-co-MMA) composites by heterocoagulation and blending with PET. J Colloid Interface Sci 308:81–92
Tarameshlou M, Jafari SH, Khonakdar HA, Fakhravar A, Farmahini-Farahani M (2010) PET-based nanocomposites made by reactive and remodified clays. Iran Polym J 19(7):521–529
Costache MC, Heidecker MJ, Manias E, Wilkie CA (2006) Preparation and characterization of poly (ethylene terephthalate)/clay nanocomposites by melt blending using thermally stable surfactants. Polym Adv Technol 17:764–771
Park SY, Kim HY, Jin FL, Park SJ (2010) Particle dispersibility improvement of polyester fibers with a new line injection. Bull Korean Chem Soc 32(9):2637–2643
Davarcioglu B (2011) Spectral characterization of non-clay minerals found in the clays (Central Anatolian-Turkey). Int J Phys Sci 6(3):511–522
Subramaniam MD, Kim IH (2015) Clays as dietary supplements for swine: a review. J Anim Sci Biotechnol 6(38):1–9
Ismadji S et al (2015) Natural clay minerals as environmental cleaning agents. Clay materials for environmental remediation. Springer, Switzerland, pp 5–37
At Thøgersen, Diplas S, Mayandi J, Finstad T, Olsen A, Watts JF, Mitome M, Bando Y (2008) An experimental study of charge distribution in crystalline and amorphous Si nanoclusters in thin silica films. J Appl Phys 103(2):024308
Thøgersen A, Selj JH, Marstein ES (2012) Oxidation effects on graded porous silicon anti-reflection coatings. J Electrochem Soc 159(5):D276–D278
Munasir Triwikantoro, Zainuri MD (2015) Synthesis of SiO2 nanopowders containing quartz and cristobalite phases from silica sands. Mater Sci-Pol 33(1):47–55
Masui J, Parvin S, Sato E, Miyashita T (2010) Preparation of organic-ceramic-metal multihybrid particles and their organized assembly. Polym J 42:142–147
Zhang WH, Fan SD, Tian W, Fan WW (2012) Polystyrene/nano-SiO2 composite microspheres fabricated by Pickering emulsion polymerization: preparation, mechanisms and thermal properties. Express Polym Lett 6(7):532–542
Go SS, Lee HC, Lee JY, Kim JH, Chung CW (2009) Experimental investigation of mortars using activated Hwangtoh. Constr Build Mater 23:1438–1445
Go SS, Chung CW, Struble LJ, Lee HC (2010) Pozzolanic activity of Hwangtoh clay. Constr Build Mater 24:2638–2645
Zhang G, Shichi T, Takagi K (2003) PET-clay hybrids with improved tensile strength. Mater Lett 57:1858–1862
Scaffaro R, Botta L, Ceraulo M, La Mantia FP (2011) Effect of kind and content of Organo-Modified clay on properties of PET nanocomposites. J Appl Polym Sci 122:384–392
Kim SH, Pyo HB, Ko SH, Ah CS, Kim AS, Kim WJ (2010) Fabrication of anionic sulfate-functionalized nanoparticles as an immunosensor by protein immobilization. Langmuir 26(10):7355–7364
Saikia B, Parthasarathy G (2010) Fourier transform infrared spectroscopic characterization of kaolinite from Assam and Meghalaya, Northeastern India. J Mod Phys 1:206–210
Janik LJ, Keeling J (1993) FT-IR partial least-square analysis of tubular halloysite in kaolin samples from the Mount Hope kaolin deposit. Clay Miner 28:365–378
Ekosse GIE (2005) Fourier transform infrared spectrophotometry and X-ray powder diffractometry as complementary techniques in characterizing clay size fraction of kaolin. J Appl Sci Environ Manag 9(2):43–48
Bordeepong S, Bhongsuwan D, Pungrassami T, Bhongsuwan T (2011) Characterization of halloysite from Thung Yai District, Nakhon Si Thammarat Province, in Southern Thailand, Songklanakarin. J. Sci. Technol 33(5):599–607
Bobos I, Duplay J, Rocha J, Gomes C (2011) Kaolinite to halloysite-7 Å transformation in kaolin deposits of São Vicente de Pereira, Portugal (2011). Clays Clay Miner 49(6):596–607
Banerjee AN, Ghosh CK, Chattopadhyay KK, Minoura H, Sarkar AK, Akiba A, Kamiya A, Endo T (2010) Low-temperature deposition of ZnO thin films on PET and glass substrates by DC-sputtering technique. Thin Solid Films 496:112–116
Abdullah M, Khairurrijal K (2008) Derivation of Scherrer relation using and approach in basic Physics course. J nano Saintek. 1(1):28–32
Ghita R et al (2011) Crystalline silicon—properties and uses. Study of SiO2/Si interface by surface technique. Intech, Croatia, pp 23–42
Lu WC, Wang CA, Nguyen V, Schmidt MW, Gordon MS, Ho KM (2003) Structures and fragmentation of small silicon oxide clusters by ab initio calculations. J Phys Chem 107:6936–6943
Barranco A, Mejías JA, Espinós JP, Caballerom A, González-Elipe RA, Yubero F (2001) Chemical stability of Sin+ species in SiO x (x < 2) thin films. J Vac Sci Technol 19(1):136–144
Acknowledgements
The authors would like to thank Dr. Lord for the preparation of the experimental setup and manuscript. This work was supported by Hansung University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, WJ., Kim, C.A. & Kim, S. Preparation and characterization of PET blended with silica–polystyrene hybrid nanocomposites. Polym. Bull. 75, 1505–1517 (2018). https://doi.org/10.1007/s00289-017-2107-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2107-y