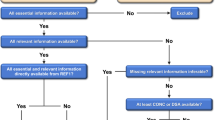
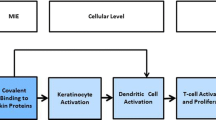
Abstract
United States regulatory and research agencies may rely upon skin sensitization test data to assess the sensitization hazards associated with dermal exposure to chemicals and products. These data are evaluated to ensure that such substances will not cause unreasonable adverse effects to human health when used appropriately. The US Consumer Product Safety Commission, the US Environmental Protection Agency, the US Food and Drug Administration, the Occupational Safety and Health Administration, the National Institute for Occupational Safety and Health, and the US Department of Defense are member agencies of the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM seeks to identify opportunities for the use of non-animal replacements to satisfy these testing needs and requirements. This review identifies the standards, test guidelines, or guidance documents that are applicable to satisfy each of these agency’s needs; the current use of animal testing and flexibility for using alternative methodologies; information needed from alternative tests to fulfill the needs for skin sensitization data; and whether data from non-animal alternative approaches are accepted by these US federal agencies.

Similar content being viewed by others
References
ASTM (2013) F 2148-13, standard practice for evaluation of delayed contact hypersensitivity using the murine local lymph node assay (LLNA). American Society for Testing and Materials, West Conshohocken
Casati S, Aschberger K, Barroso J, Casey W, Delgado I, Kim TS, Kleinstreuer N, Kojima H, Lee JK, Lowit A, Park HK, Regimbald-Krnel MJ, Strickland J, Whelan M, Yang Y, Zuang V (2018) Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: position of the international cooperation on alternative test methods. Arch Toxicol 92(2):611–617
Casey W (2016) Advances in the development and validation of test methods in the United States. Toxicol Res 32(1):9–14
CPSC (2012) Recommended procedures regarding the CPSC’s policy on animal testing. https://www.cpsc.gov/business--manufacturing/testing-certification/recommended-procedures-regarding-the-cpscs-policy-on-animal-testing Accessed 10 July 2018
CPSC (2013) CPSC staff’s strong sensitizer guidance document. https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_strongsensitizerguidance.pdf. Accessed 10 July 2018
CPSC (2017a) Art materials. Labeling of hazardous art materials act. 16 CFR 1500.14(b)(8)
CPSC (2017b) Certain statutory definitions interpreted, supplemented, or provided with alternatives. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)
CPSC (2017c) Definition of sensitizer. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)(i)
CPSC (2017d) Listing of “strong sensitizer” substances. Federal Hazardous Substances Act. 16 CFR 1500.13
CPSC (2017e) Significant potential for causing hypersensitivity. Federal Hazardous Substances Act. 16 CFR 1500.3(c)(5)(ii)
CPSC (2017f) Statement on animal testing policy. Federal Hazardous Substances Act. 16 CFR 1500.232
Daniel AB, Strickland J, Allen D, Casati S, Zuang V, Barroso J, Whelan M, Regimbald-Krnel MJ, Kojima H, Nishikawa A, Park HK, Lee JK, Kim TS, Delgado I, Rios L, Yang Y, Wang G, Kleinstreuer N (2018) International regulatory requirements for skin sensitization testing. Regul Toxicol Pharmacol 95:52–65
de Ávila R, Teixeira G, Veloso D, Moreira L, Lima E, Valadares M (2017) In vitro assessment of skin sensitization, photosensitization and phototoxicity potential of commercial glyphosate-containing formulations. Toxicol In Vitro 45(Pt 3):385–392
ECHA (2017) Guidance on information requirements and chemical safety assessment. Chapter R.7a: endpoint specific guidance (version 6.0). European Chemicals Agency (ECHA), Helsinki
EPA (2003) Health effects test guidelines: OPPTS 870.2600—skin sensitization. US Environmental Protection Agency, Washington, DC
EPA (2011) Expansion of the traditional local lymph node assay for the assessment of dermal sensitization potential of end use pesticide products; and adoption of a “reduced” protocol for the traditional LLNA (limit dose). Office of Pesticide Programs, Washington, DC
EPA (2012) Guidance for waiving or bridging of mammalian acute toxicity tests for pesticides and pesticide products (acute oral, acute dermal, acute inhalation, primary eye, primary dermal, and dermal sensitization) (March 1, 2012). Office of Pesticide Programs, Washington, DC
EPA (2016) Process for evaluating and implementing alternative approaches to traditional in vivo acute toxicity studies for FIFRA regulatory use. Office of Pesticide Programs, Washington, DC
EPA (2017) Summary of the Federal Insecticide, Fungicide, and Rodenticide Act. https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act. Accessed 10 July 2018
EPA (2018a) Interim science policy: use of alternative approaches for skin sensitization as a replacement for laboratory animal testing. Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs, Office of Pollution Prevention and Toxics, Washington, DC
EPA (2018b) Strategic plan to promote the development and implementation of alternative methods within the TSCA program. Office of Chemical Safety and Pollution Prevention, Washington, DC
EPA (2018c) Strategic vision for adopting 21st century science methodologies. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/strategic-vision-adopting-21st-century-science Accessed 10 July 2018
FDA (1975) Cosmetic products: warning statements/package labels. Fed Reg 40(42):8912–8916
FDA (1999a) Guidance for industry—skin irritation and sensitization testing of generic transdermal drug products (draft guidance). http://www.pharmanet.com.br/pdf/2887dft.pdf. Accessed 31 July 2018
FDA (1999b) Guidance for industry and FDA reviewers/staff: premarket notification [501(k)] submissions for testing for skin sensitization to chemicals in natural rubber products. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073793.pdf. Accessed 31 July 2018
FDA (2002) Guidance for industry: immunotoxicology evaluation of investigational new drugs. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM079239.pdf. Accessed 31 July 2018
FDA (2008) Guidance for industry and FDA staff: medical glove guidance manual. https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm428191.pdf. Accessed 31 July 2018
FDA (2016) Guidance for Industry and Food and Drug Administration staff: use of international standard ISO 10993-1, “Biological evaluation of medical devices—part 1: evaluation and testing within a risk management process. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm348890.pdf. Accessed 31 July 2018
FDA (2018a) About the Center for Devices and Radiologic Health. https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cdrh/. Accessed 10 July 2018
FDA (2018b) About the Center for Drug Evaluation and Research. In. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/default.htm. Accessed 10 July 2018
FDA (2018c) About the Center for Veterinary Medicine (CVM). In. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CVM/default.htm. Accessed 26 July 2018
FDA (2018d) CBER vision & mission. In. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm122878.htm. Accessed 26 July 2018
FDA (2018e) CFSAN—what we do. https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CFSAN/WhatWeDo/default.htm. Accessed 10 July 2018
FDA (2018f) Product testing. https://www.fda.gov/Cosmetics/ScienceResearch/ProductTesting. Accessed 10 July 2018
ISO (2010) ISO 10993-10: Biological evaluation of medical devices—part 10: tests for irritation and skin sensitization. International Organization for Standardization, Geneva
JRC (2017) EURL ECVAM recommendation on the use of non-animal approaches for skin sensitisation testing. Publications Office of the European Union, Luxembourg
JRC (2018) Database on alternative methods (DB-ALM). https://eurl-ecvam.jrc.ec.europa.eu/databases/database-on-alternative-methods-db-alm. Accessed 26 July 2018
Kleinstreuer N, Hoffmann S, Alepee N et al (2018) Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 48(5):359–374
Murphy K, Travers P, Walport M, Janeway C (2012) Janeway’s immunobiology, 8th edn. Garland Science, New York
NIEHS (2018) A strategic roadmap for establishing new approaches to evaluate the safety of chemicals and medical products in the United States. https://www.ntp.niehs.nih.gov/go/natl-strategy. Accessed 10 July 2018
NIOSH (2009) A strategy for assigning new NIOSH skin notations. Current intelligence bulletin 61. DHHS (NIOSH) Publication No. 2009-147
NTP (2018) Alternative methods accepted by US agencies. https://ntp.niehs.nih.gov/pubhealth/evalatm/accept-methods/index.html. Accessed 10 July 2018
OECD (1992) Test no. 406. Skin sensitisation. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010a) Test no. 429. Skin sensitisation: local lymph node assay. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010b) Test no. 442A. Skin sensitization: local lymph node assay: DA. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2010c) Test no. 442B. Skin sensitization: local lymph node assay: BrdU-ELISA. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2012a) OECD series on testing and assessment no. 168. The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Part 2: use of the AOP to develop chemical categories and integrated assessment and testing approaches. OECD Publishing, Paris
OECD (2012b) OECD series on testing and assessment no. 168. The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Part 1: scientific assessment. OECD Publishing, Paris
OECD (2015) Test no. 442C. In chemico skin sensitization: direct peptide reactivity assay (DPRA). OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2016a) OECD series on testing and assessment no. 256. Guidance document on the reporting of defined approaches and individual information sources to be used within integrated approaches to testing and assessment (IATA) for skin sensitisation. OECD Publishing, Paris
OECD (2016b) OECD series on testing and assessment no. 256. Guidance document on the reporting of defined approaches and individual information sources to be used within integrated approaches to testing and assessment (IATA) for skin sensitisation. Annex 1. OECD Publishing, Paris
OECD (2017a) Test no. 442E. In vitro skin sensitisation. In vitro skin sensitisation assays addressing the key event on activation of dendritic cells on the adverse outcome pathway for skin sensitisation. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OECD (2017b) Work plan for the test guidelines program (TGP) as of August 2017. http://www.oecd.org/chemicalsafety/testing/TGP%20work%20plan_August%202017.pdf. Accessed 31 July 2018
OECD (2018a) OECD guidelines for the testing of chemicals, section 4, health effects. https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788. Accessed 26 July 2018
OECD (2018b) OECD test guidelines programme. http://www.oecd.org/chemicalsafety/testing/oecd-guidelines-testing-chemicals-related-documents.htm. Accessed 26 July 2018
OECD (2018c) Test no. 442D. In vitro skin sensitisation: ARE-Nrf2 luciferase test method. OECD guidelines for the testing of chemicals, section 4: health effects. OECD Publishing, Paris
OSHA (2016) Hazard communication: hazard classification guidance for manufacturers, importers, and employees. Occupational Safety and Health Administration, US Department of Labor Publication: OSHA 3844-02. https://www.osha.gov/Publications/OSHA3844.pdf. Accessed 10 July 2018
OSHA (2017) Hazard communication. Occupational Safety and Health Act. 29 CFR 1910.1200
Rovida C, Alepee N, Api AM et al (2015) Integrated testing strategies (ITS) for safety assessment. ALTEX 32(1):25–40
Settivari R, Gehen S, Amado R, Visconti N, Boverhof D, Carney E (2015) Application of the KeratinoSens™ assay for assessing the skin sensitization potential of agrochemical active ingredients and formulations. Regul Toxicol Pharmacol 72(2):350–360
Strickland J, Zang Q, Kleinstreuer N, Paris M, Lehmann DM, Choksi N, Matheson J, Jacobs A, Lowit A, Allen D, Casey W (2016) Integrated decision strategies for skin sensitization hazard. J Appl Toxicol 36(9):1150–1162
UN (2009) Globally harmonized system of classification and labelling of chemicals, vol ST/SG/AC.10/30/Rev.3, 3rd rev. edn. United Nations, New York
Varsho B, Carathers M, Vij P, DeGeorge G (2017) The human cell line activation test (h-CLAT) for assessment of dermal sensitization potency of commercially available mixtures and the OECD proficiency chemicals. ALTEX Proc 5(1):40
Acknowledgements
The authors thank Drs. B. Alex Merrick and Kristen Ryan for their thoughtful critical review of this manuscript, and Ms. Catherine Sprankle for editorial review. This project was funded in part with federal funds from the National Institute of Environmental Health Sciences, National Institutes of Health under Contract No. HHSN273201500010C to ILS in support of the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods.
Disclaimer
This article may be the work product of an employee or group of employees of the US CPSC, DoD, EPA, FDA, NIEHS, NIH, NIOSH, OSHA, or other organizations. However, the statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of these organizations or the United States government. ILS staff provides technical support for NICEATM, but do not represent NIEHS, NTP, or the official positions of any federal agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Strickland, J., Daniel, A.B., Allen, D. et al. Skin sensitization testing needs and data uses by US regulatory and research agencies. Arch Toxicol 93, 273–291 (2019). https://doi.org/10.1007/s00204-018-2341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2341-6